Table Of Standard Reduction Potentials - Vaxasoftware
ADVERTISEMENT
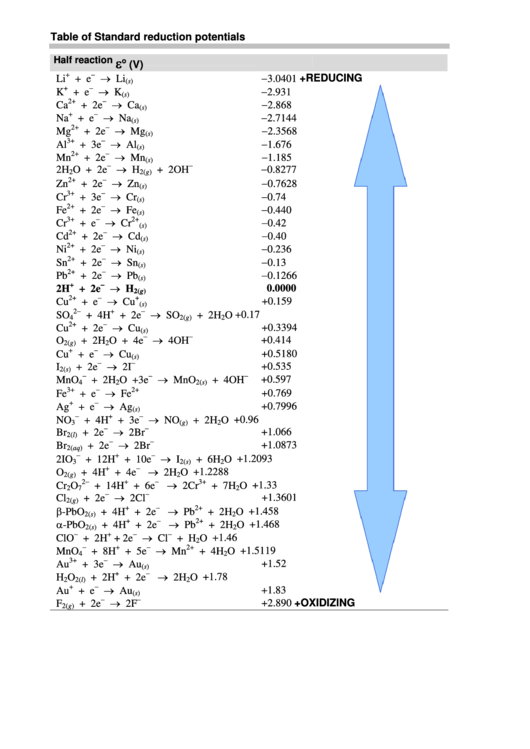
Table of Standard reduction potentials
ε
Half reaction
o
(V)
−
→ Li
−3.0401
+
+REDUCING
Li
+ e
(s)
−
→ K
−2.931
+
K
+ e
(s)
−
→ Ca
−2.868
2+
Ca
+ 2e
(s)
−
→ Na
−2.7144
+
Na
+ e
(s)
−
→ Mg
−2.3568
2+
Mg
+ 2e
(s)
−
→ Al
−1.676
3+
Al
+ 3e
(s)
−
→ Mn
−1.185
2+
Mn
+ 2e
(s)
−
−
→ H
−0.8277
2H
O + 2e
+ 2OH
2
2(g)
−
→ Zn
−0.7628
2+
Zn
+ 2e
(s)
−
→ Cr
−0.74
3+
Cr
+ 3e
(s)
−
→ Fe
−0.440
2+
Fe
+ 2e
(s)
−
→ Cr
−0.42
3+
2+
Cr
+ e
(s)
−
→ Cd
−0.40
2+
Cd
+ 2e
(s)
−
→ Ni
−0.236
2+
Ni
+ 2e
(s)
−
→ Sn
−0.13
2+
Sn
+ 2e
(s)
−
→ Pb
−0.1266
2+
Pb
+ 2e
(s)
−
→ H
+
0.0000
2H
+ 2e
2(g)
−
→ Cu
2+
+
+0.159
Cu
+ e
(s)
−
→ SO
2−
+
+0.17
SO
+ 4H
+ 2e
+ 2H
O
4
2(g)
2
−
→ Cu
2+
+0.3394
Cu
+ 2e
(s)
−
−
→ 4OH
+0.414
O
+ 2H
O + 4e
2(g)
2
−
→ Cu
+
+0.5180
Cu
+ e
(s)
−
−
→ 2I
+0.535
I
+ 2e
2(s)
−
−
−
→ MnO
+0.597
MnO
+ 2H
O +3e
+ 4OH
4
2
2(s)
−
→ Fe
3+
2+
+0.769
Fe
+ e
−
→ Ag
+
+0.7996
Ag
+ e
(s)
−
−
→ NO
+
+0.96
NO
+ 4H
+ 3e
+ 2H
O
3
(g)
2
−
−
→ 2Br
+1.066
Br
+ 2e
2(l)
−
−
→ 2Br
+1.0873
Br
+ 2e
2(aq)
−
−
→ I
+
+1.2093
2IO
+ 12H
+ 10e
+ 6H
O
3
2(s)
2
−
→ 2H
+
+1.2288
O
+ 4H
+ 4e
O
2(g)
2
−
→ 2Cr
2−
+
3+
+1.33
Cr
O
+ 14H
+ 6e
+ 7H
O
2
7
2
−
−
→ 2Cl
+1.3601
Cl
+ 2e
2(g)
−
β-PbO
→ Pb
+
2+
+1.458
+ 4H
+ 2e
+ 2H
O
2(s)
2
−
α-PbO
→ Pb
+
2+
+1.468
+ 4H
+ 2e
+ 2H
O
2(s)
2
−
−
−
→ Cl
+
+1.46
ClO
+ 2H
+ 2e
+ H
O
2
−
−
→ Mn
+
2+
+1.5119
MnO
+ 8H
+ 5e
+ 4H
O
4
2
−
→ Au
3+
+1.52
Au
+ 3e
(s)
−
→ 2H
+
+1.78
H
O
+ 2H
+ 2e
O
2
2(l)
2
−
→ Au
+
+1.83
Au
+ e
(s)
−
−
→ 2F
+2.890
+OXIDIZING
F
+ 2e
2(g)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1