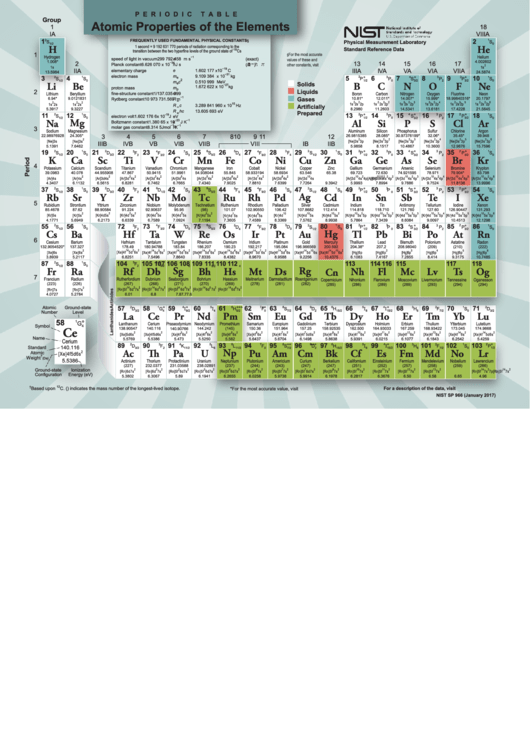
Periodic Table Atomic Properties Of The Elements
ADVERTISEMENT
P E R I O D I C
T A B L E
Group
Atomic Properties of the Elements
1
18
IA
VIIIA
1
2
2
FREQUENTLY USED FUNDAMENTAL PHYSICAL CONSTANTS
S
§
Physical Measurement Laboratory
1/2
H
1 second = 9 192 631 770 periods of radiation corresponding to the
He
Standard Reference Data
transition between the two hyperfine levels of the ground state of
133
Cs
1
For the most accurate
§
Hydrogen
Helium
−1
speed of light in vacuum
299 792 458 m s
(exact)
c
values of these and
1.008*
4.002602
2
13
14
15
16
17
−34
Planck constant
h
6.626 070
10
J s
(
ħ
/2 )
other constants, visit
x
1s
−19
pml.nist.gov/constants
IIA
IIIA
IVA
VA
VIA
VIIA
elementary charge
e
1.602 177
10
C
x
13.5984
24.5874
−31
electron mass
m
9.109 384
x
10
kg
3
4
5
6
7
8
9
10
1
2
e
°
°
°
S
S
2
1/2
0
m
c
0.510 999 MeV
Li
Be
Solids
B
C
N
O
F
Ne
e
−27
proton mass
1.672 622
x
10
kg
m
p
2
Liquids
Lithium
Beryllium
fine-structure constant
1/137.035 999
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
−1
Gases
6.94*
9.0121831
10.81*
12.011*
14.007*
15.999*
18.99840316*
20.1797
Rydberg constant
R
10 973 731.569 m
2
2
2
1s
2s
1s
2s
15
R c
3.289 841 960
10
Hz
Artificially
x
5.3917
9.3227
8.2980
11.2603
14.5341
13.6181
17.4228
21.5645
R
hc
13.605 693 eV
Prepared
11
12
13
14
15
16
17
18
2
1
°
°
°
S
S
-19
electron volt
eV
1.602 176 6 x 10
J
0
1/2
Mg
Na
Al
Si
P
S
Cl
Ar
−23
−1
Boltzmann constant
k
1.380 65
10
J K
x
−1
−1
molar gas constant
R
8.314 5 J mol
K
3
Sodium
Magnesium
Aluminum
Silicon
Phosphorus
Sulfur
Chlorine
Argon
3
4
5
6
7
8
9
10
11
12
22.98976928
24.305*
26.9815385
28.085*
30.97376199*
32.06*
35.45*
39.948
2
2
2
2
2
3
2
4
2
5
2
6
[Ne]3s
[Ne]3s
[Ne]3s
3p
[Ne]3s
3p
[Ne]3s
3p
[Ne]3s
3p
[Ne]3s
3p
[Ne]3s
3p
IIIB
IVB
VB
VIB
VIIB
VIII
IB
IIB
5.1391
7.6462
5.9858
8.1517
10.4867
10.3600
12.9676
15.7596
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
2
1
2
3
4
7
6
5
4
°
°
°
S
S
D
F
F
S
S
D
F
1/2
0
3/2
2
3/2
3
5/2
4
9/2
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Br
Kr
4
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
Gallium
Germanium
Arsenic
Selenium
Bromine
Krypton
39.0983
40.078
44.955908
47.867
50.9415
51.9961
54.938044
55.845
58.933194
58.6934
63.546
65.38
69.723
72.630
74.921595
78.971
79.904*
83.798
2
2
2
2
3
2
5
5
2
6
2
7
2
8
2
10
10
2
10
2
10
2
2
10
2
3
10
2
4
10
2
5
10
2
6
[Ar]4s
[Ar]4s
[Ar]3d4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
[Ar]3d
4s
4p
[Ar]3d
4s
4p
[Ar]3d
4s
4p
[Ar]3d
4s
4p
[Ar]3d
4s
4p
[Ar]3d
4s
4p
4.3407
6.1132
6.5615
6.8281
6.7462
6.7665
7.4340
7.9025
7.8810
7.6399
7.7264
9.3942
5.9993
7.8994
9.7886
9.7524
11.8138
13.9996
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
2
1
2
3
6
7
6
5
4
1
2
°
°
S
S
D
D
S
S
F
F
S
F
S
1/2
0
3/2
2
1/2
3
5/2
5
9/2
0
1/2
Ag
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Cd
In
Sn
Sb
Te
I
Xe
5
Rubidium
Strontium
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
Silver
Cadmium
Indium
Tin
Antimony
Tellurium
Iodine
Xenon
85.4678
87.62
88.90584
91.224
92.90637
95.95
(98)
101.07
102.90550
106.42
107.8682
112.414
114.818
118.710
121.760
127.60
126.90447
131.293
2
2
2
2
4
5
5
2
10
10
10
2
10
2
10
2
2
10
2
3
10
2
4
10
2
5
10
2
6
7
8
[Kr]5s
[Kr]4d5s
[Kr]4d
5s
[Kr]4d
5s
[Kr]4d
5s
[Kr]4d
5s
[Kr]4d
5s
5p
[Kr]4d
5s
5p
[Kr]4d
5s
5p
[Kr]4d
5s
5p
[Kr]5s
[Kr]4d
5s
[Kr]4d
5s
[Kr]4d
[Kr]4d
5s
[Kr]4d
5s
[Kr]4d
5s
5p
[Kr]4d
5s
5p
4.1771
5.6949
6.2173
6.6339
6.7589
7.0924
7.1194
7.3605
7.4589
8.3369
7.5762
8.9938
5.7864
7.3439
8.6084
9.0097
10.4513
12.1298
55
56
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
2
1
3
4
5
6
5
4
3
2
°
°
°
S
S
F
F
D
S
D
F
D
S
1/2
0
2
3/2
0
5/2
4
9/2
3
1/2
Hg
Cs
Ba
Hf
Ta
W
Re
Os
Ir
Pt
Au
Tl
Pb
Bi
Po
At
Rn
6
Cesium
Barium
Hafnium
Tantalum
Tungsten
Rhenium
Osmium
Iridium
Platinum
Gold
Mercury
Thallium
Lead
Bismuth
Polonium
Astatine
Radon
132.9054520*
137.327
178.49
180.94788
183.84
186.207
190.23
192.217
195.084
196.966569
200.592
204.38*
207.2
208.98040
(209)
(210)
(222)
2
14
2
2
14
3
2
14
4
2
14
5
2
14
6
2
14
7
2
14
9
14
10
14
10
2
2
3
4
5
6
[Xe]6s
[Xe]6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Xe]4f
5d
6s
[Hg]6p
[Hg]6p
[Hg]6p
[Hg]6p
[Hg]6p
[Hg]6p
3.8939
5.2117
6.8251
7.5496
7.8640
7.8335
8.4382
8.9670
8.9588
9.2256
10.4375
6.1083
7.4167
7.2855
8.414
9.3175
10.7485
87
88
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
2
1
3
4
S
S
F
F
1 /2
0
2
3/2
0
5/2
4
Sg
Rg
Fr
Ra
Rf
Db
Bh
Hs
Mt
Ds
Nh
Fl
Mc
Lv
Ts
Og
Cn
7
Francium
Radium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Meitnerium
Darmstadtium
Roentgenium
Copernicium
Nihonium
Flerovium
Moscovium
Livermorium
Tennessine
Oganesson
(223)
(226)
(267)
(268)
(271)
(270)
(269)
(278)
(281)
(282)
(285)
(286)
(289)
(289)
(293)
(294)
(294)
2
14
2
2
14
3
2
14
4
2
14
5
2
14
6
2
[Rn]5f
6d
7s
[Rn]5f
6d
7s
[Rn]5f
6d
7s
[Rn]5f
6d
7s
[Rn]5f
6d
7s
[Rn]7s
[Rn]7s
4.0727
5.2784
6.01
6.8
7.8
7.7
7.6
Atomic
Ground-state
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
2
1
°
4
°
5
6
°
7
8
°
9
°
6
°
5
4
°
3
2
°
1
2
D
G
I
I
H
F
S
D
H
I
I
H
F
S
D
Number
Level
3/2
4
9/2
4
5/2
0
7/2
2
15/2
8
15/2
6
7/2
0
3/2
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
58
°
1
G
Lanthanum
Cerium
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
Ytterbium
Lutetium
Symbol
4
Ce
138.90547
140.116
140.90766
144.242
(145)
150.36
151.964
157.25
158.92535
162.500
164.93033
167.259
168.93422
173.045
174.9668
2
2
3
2
4
2
5
2
6
2
7
2
7
2
9
2
10
2
11
2
12
2
13
2
14
2
14
2
[Xe]5d6s
[Xe]4f5d6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
5d6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
6s
[Xe]4f
5d6s
Name
5.5769
5.5386
5.473
5.5250
5.582
5.6437
5.6704
6.1498
5.8638
5.9391
6.0215
6.1077
6.1843
6.2542
5.4259
Cerium
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
2
3
4
5
°
6
7
8
°
9
°
6
°
5
4
°
3
2
°
1
°
D
K
F
I
H
F
F
L
L
S
D
H
I
S
Standard
140.116
3/2
2
11/2
6
11/2
0
7/2
2
15/2
8
15/2
6
7/2
0
Np
Ac
Pa
U
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
Atomic
2
[Xe]4f5d6s
†
Weight
(Da)
5.5386
Actinium
Thorium
Protactinium
Uranium
Neptunium
Plutonium
Americium
Curium
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
(227)
232.0377
231.03588
238.02891
(237)
(244)
(243)
(247)
(247)
(251)
(252)
(257)
(258)
(259)
(266)
Ground-state
Ionization
2
2
2
2
2
3
2
4
2
6
2
7
2
7
2
9
2
10
2
11
2
12
2
13
2
14
2
14
2
[Rn]6d7s
[Rn]6d
7s
[Rn]5f
6d7s
[Rn]5f
6d7s
[Rn]5f
6d7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
6d7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
7s
[Rn]5f
7s
7p
Configuration
Energy (eV)
5.3802
6.3067
5.89
6.1941
6.2655
6.0258
5.9738
5.9914
6.1978
6.2817
6.3676
6.50
6.58
6.65
4.96
†
12
For a description of the data, visit pml.nist.gov/data
Based upon
C. () indicates the mass number of the longest-lived isotope.
*For the most accurate value, visit .
NIST SP 966 (January 2017)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








