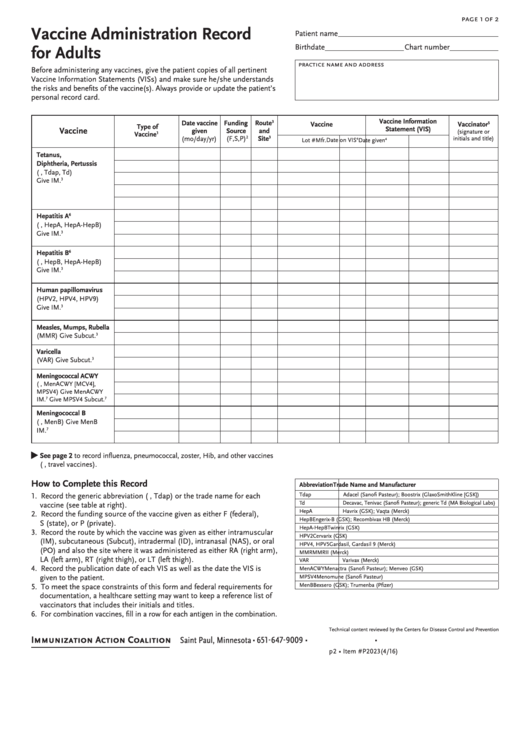
Vaccine Administration Record For Adults
ADVERTISEMENT
page 1 0f 2
Vaccine Administration Record
Patient name
for Adults
Birthdate
Chart number
practice name and address
Before administering any vaccines, give the patient copies of all pertinent
Vaccine Information Statements (VISs) and make sure he/she understands
the risks and benefits of the vaccine(s). Always provide or update the patient’s
personal record card.
Vaccine Information
Date vaccine
Funding
Route
3
Vaccine
Vaccinator
5
Type of
Statement (VIS)
Vaccine
given
Source
and
(signature or
Vaccine
1
(mo/day/yr)
(F,S,P)
2
Site
3
initials and title)
Lot #
Mfr.
Date on VIS
4
Date given
4
Tetanus,
Diphtheria, Pertussis
(e.g., Tdap, Td)
Give IM.
3
Hepatitis A
6
(e.g., HepA, HepA-HepB)
Give IM.
3
Hepatitis B
6
(e.g., HepB, HepA-HepB)
Give IM.
3
Human papillomavirus
(HPV2, HPV4, HPV9)
Give IM.
3
Measles, Mumps, Rubella
(MMR) Give Subcut.
3
Varicella
(VAR) Give Subcut.
3
Meningococcal ACWY
(e.g., MenACWY [MCV4],
MPSV4) Give MenACWY
IM.
7
Give MPSV4 Subcut.
7
Meningococcal B
(e.g., MenB) Give MenB
IM.
7
•
See page 2 to record influenza, pneumococcal, zoster, Hib, and other vaccines
(e.g., travel vaccines).
How to Complete this Record
Abbreviation
Trade Name and Manufacturer
1. Record the generic abbreviation (e.g., Tdap) or the trade name for each
Tdap
Adacel (Sanofi Pasteur); Boostrix (GlaxoSmithKline [GSK])
Td
Decavac, Tenivac (Sanofi Pasteur); generic Td (MA Biological Labs)
vaccine (see table at right).
HepA
Havrix (GSK); Vaqta (Merck)
2. Record the funding source of the vaccine given as either F (federal),
HepB
Engerix-B (GSK); Recombivax HB (Merck)
S (state), or P (private).
HepA-HepB
Twinrix (GSK)
3. Record the route by which the vaccine was given as either intramuscular
HPV2
Cervarix (GSK)
(IM), subcutaneous (Subcut), intradermal (ID), intranasal (NAS), or oral
HPV4, HPV5
Gardasil, Gardasil 9 (Merck)
(PO) and also the site where it was administered as either RA (right arm),
MMR
MMRII (Merck)
LA (left arm), RT (right thigh), or LT (left thigh).
VAR
Varivax (Merck)
4. Record the publication date of each VIS as well as the date the VIS is
MenACWY
Menactra (Sanofi Pasteur); Menveo (GSK)
given to the patient.
MPSV4
Menomune (Sanofi Pasteur)
5. To meet the space constraints of this form and federal requirements for
MenB
Bexsero (GSK); Trumenba (Pfizer)
documentation, a healthcare setting may want to keep a reference list of
vaccinators that includes their initials and titles.
6. For combination vaccines, fill in a row for each antigen in the combination.
Technical content reviewed by the Centers for Disease Control and Prevention
651 - 647 - 9009
Immunization Action Coalition
Saint Paul, Minnesota
•
•
•
/catg.d/p2023.pdf
Item #P2023 (4/16)
•
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Medical
 1
1 2
2 3
3 4
4








