The Periodic Chart Of Atoms Page 2
ADVERTISEMENT
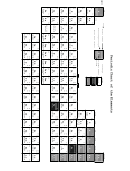
19. The number under the symbol in each square indicates the Average Atomic __________.
20. Find the symbol O , which denotes the element _______________.
21. Oxygen’s __________ __________ is 8.
22. Oxygen’s average atomic mass is __________.
23. Cu denotes the element _______________.
24. Its average atomic mass is _______________.
25. Hydrogen has an Average Atomic Mass of __________.
26. Lead (Pb) has an Average Atomic Mass of __________.
27. Mercury (Hg) has an Average Atomic Mass of __________.
28. The atomic number of Carbon is __________.
29. The Average Atomic Mass of Carbon is __________.
30. The atomic Number of Calcium (Ca) is __________.
31. The Average Atomic Mass of Ca is __________.
32. The __________ __________ __________ of Gold (Au) is 196.966.
33. Its Atomic __________ is 79.
34. The _______________ is an atomic particle which is neutral.
35. The nucleus contains two types of particles: the plus particle called a(n) ______________
and a neutral particle called a(n) _______________.
36. Oxygen has an Atomic __________ of 8 and an Average Atomic Mass of __________.
37. Therefore, oxygen has _____ protons in its nucleus, _____ electrons in its shells, and _____
neutrons also in its nucleus.
38. The average atomic mass of silver is _____. The atomic number of silver is _____. The
difference between the average atomicmass, rounded off to the nearest whole number and
the atomic number is __________. This means that an atom of silver has 61 neutrons.
39. An atom of silver has _______ neutrons, _____ electrons and _____ protons.
40. An atom of bismuth has _____ protons, _____ electrons and _____ neutrons.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3