Organic Chemistry Sheet
ADVERTISEMENT
Chapter 13
Organic Chemistry
1. What is the difference between saturated and unsaturated hydrocarbons?
Alkanes contain only single bonds and are saturated hydrocarbons because they cannot react with hydrogen
to produce more C-H bonds. However, multiple (double or triple) bonds are unsaturated because an H
2
molecule can add across a multiple bond to form two C-H bonds. For example, ethene can be hydrogenated to
ethane: H
C=CH
+ H
→ H
C-CH
. Thus, alkenes are unsaturated. Molecules with many double bonds
2
2
2
3
3
(polyenes) are said to be polyunsaturated.
3. How do condensed structures and skeletal structures differ?
C-H bonds are not shown explicitly in either. However, the carbon and hydrogen atoms are shown in a
condensed structure, whereas only the C-C bonds are shown in a skeletal structure. The condensed and
and ⎯, respectively.
skeletal structures of ethane are: H
C-CH
3
3
5. Why are alkenes more reactive than alkanes? Are alkenes Lewis acids or Lewis bases? Explain.
The π electrons in alkenes are Lewis bases, which is why alkenes are more reactive than alkanes.
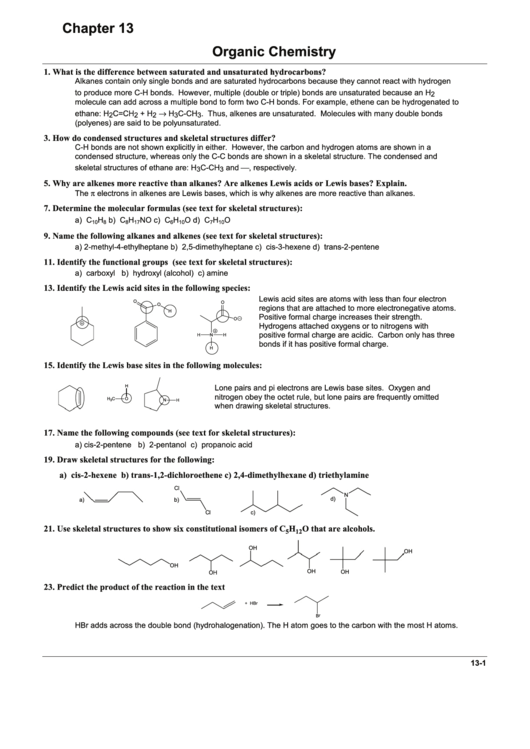
7. Determine the molecular formulas (see text for skeletal structures):
a) C
H
b) C
H
NO
c) C
H
O
d) C
H
O
10
8
8
17
6
10
7
10
9. Name the following alkanes and alkenes (see text for skeletal structures):
a) 2-methyl-4-ethylheptane
b) 2,5-dimethylheptane
c) cis-3-hexene
d) trans-2-pentene
11. Identify the functional groups (see text for skeletal structures):
a) carboxyl
b) hydroxyl (alcohol)
c) amine
13. Identify the Lewis acid sites in the following species:
Lewis acid sites are atoms with less than four electron
O
O
O
regions that are attached to more electronegative atoms.
H
Positive formal charge increases their strength.
O
Hydrogens attached oxygens or to nitrogens with
positive formal charge are acidic. Carbon only has three
H
N
H
bonds if it has positive formal charge.
H
15. Identify the Lewis base sites in the following molecules:
H
Lone pairs and pi electrons are Lewis base sites. Oxygen and
nitrogen obey the octet rule, but lone pairs are frequently omitted
H
C
O
N
H
3
when drawing skeletal structures.
17. Name the following compounds (see text for skeletal structures):
a) cis-2-pentene
b) 2-pentanol
c) propanoic acid
19. Draw skeletal structures for the following:
a) cis-2-hexene
b) trans-1,2-dichloroethene
c) 2,4-dimethylhexane
d) triethylamine
Cl
N
d)
a)
b)
c)
Cl
21. Use skeletal structures to show six constitutional isomers of C
H
O that are alcohols.
5
12
OH
OH
OH
OH
OH
OH
23. Predict the product of the reaction in the text
+ HBr
Br
HBr adds across the double bond (hydrohalogenation). The H atom goes to the carbon with the most H atoms.
13-1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








