Electrons And The Periodic Table Worksheet
ADVERTISEMENT
Name:
Skill Sheet 18-B
Electrons and the Periodic Table
What do electrons have to do with the periodic table? In this skill sheet, you will learn how
electrons are organized in the energy levels that orbit the nucleus of an atom. You will also
discover the relationship between electrons and the organization of the periodic table.
1. How do you describe the location of an electron?
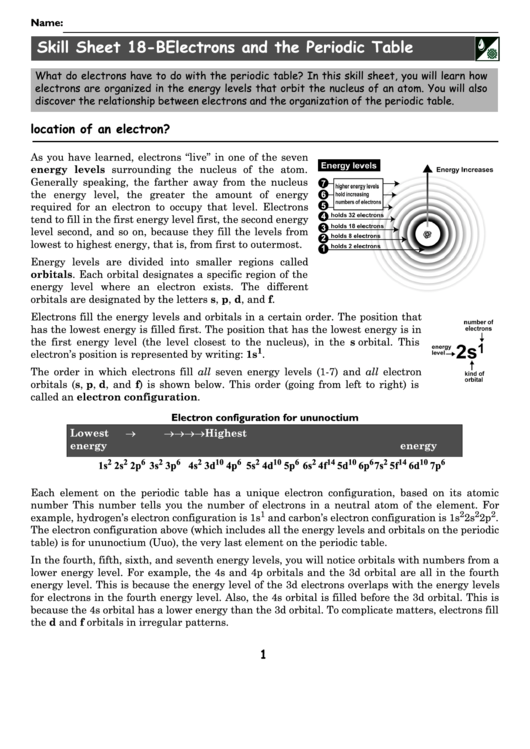
As you have learned, electrons “live” in one of the seven
energy levels surrounding the nucleus of the atom.
Generally speaking, the farther away from the nucleus
the energy level, the greater the amount of energy
required for an electron to occupy that level. Electrons
tend to fill in the first energy level first, the second energy
level second, and so on, because they fill the levels from
lowest to highest energy, that is, from first to outermost.
Energy levels are divided into smaller regions called
orbitals. Each orbital designates a specific region of the
energy level where an electron exists. The different
orbitals are designated by the letters s, p, d, and f.
Electrons fill the energy levels and orbitals in a certain order. The position that
has the lowest energy is filled first. The position that has the lowest energy is in
the first energy level (the level closest to the nucleus), in the s orbital. This
1
electron’s position is represented by writing: 1s
.
The order in which electrons fill all seven energy levels (1-7) and all electron
orbitals (s, p, d, and f) is shown below. This order (going from left to right) is
called an electron configuration.
Electron configuration for ununoctium
Lowest
→
→
→
→
→
Highest
energy
energy
2
2
6
2
6
2
10
6
2
10
6
2
14
10
6
2
14
10
6
1s
2s
2p
3s
3p
4s
3d
4p
5s
4d
5p
6s
4f
5d
6p
7s
5f
6d
7p
Each element on the periodic table has a unique electron configuration, based on its atomic
number This number tells you the number of electrons in a neutral atom of the element. For
1
2
2
2
example, hydrogen’s electron configuration is 1s
and carbon’s electron configuration is 1s
2s
2p
.
The electron configuration above (which includes all the energy levels and orbitals on the periodic
table) is for ununoctium (Uuo), the very last element on the periodic table.
In the fourth, fifth, sixth, and seventh energy levels, you will notice orbitals with numbers from a
lower energy level. For example, the 4s and 4p orbitals and the 3d orbital are all in the fourth
energy level. This is because the energy level of the 3d electrons overlaps with the energy levels
for electrons in the fourth energy level. Also, the 4s orbital is filled before the 3d orbital. This is
because the 4s orbital has a lower energy than the 3d orbital. To complicate matters, electrons fill
the d and f orbitals in irregular patterns.
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








