Nuclear Magnetic Resonance Spectroscopy With Answers Page 15
ADVERTISEMENT
375
CHAPTER 16
13
16.33. This compound will exhibit three signals in its
C NMR spectrum:
16.34.
a) 2
b) 4
c) 4
d) 2
e) 2
f) 5
16.35.
a) 4
b) 6
c) 6
d) 4
e) 2
f) 4
13
16.36. The first compound will have five signals in its
C NMR spectrum, while the
second compound will have seven signals.
16.37.
triplet
O
CH
H H
3
doublet
H
C
CH
3
3
H
C
H
3
H H H H
H
C
3
septet of triplets
or triplet of septets
singlet
(multiplet)
doublet
triplet
16.38.
13
a) The first compound will have four signals in its
C NMR spectrum, while the second
compound will have twelve signals.
1
The first compound will have two signals in its
H NMR spectrum, while the second
compound will have eight signals.
b) The first compound is a meso compound. Two of the protons are enantiotopic (the
protons that are alpha to the chlorine atoms) and are therefore chemically equivalent.
1
The first compound will only have two signals in its
H NMR spectrum, while the second
compound will have three signals. For a similar reason, first compound will only have
13
two signals in its
C NMR spectrum, while the second compound will have three signals.
13
c) The
C NMR spectrum of the second compound will have one more signal than the
13
1
C NMR spectrum of the other first compound. The
H NMR spectra will differ in the
following way: the first compound will have a singlet somewhere between 2 and 5 ppm
with an integration of 1, while the second compound will have a singlet at approximately
3.4 ppm with an integration of 3.
13
d) The first compound will have three signals in its
C NMR spectrum, while the second
compound will have five signals.
1
The first compound will have two signals in its
H NMR spectrum, while the second
compound will have four signals.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6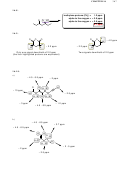 7
7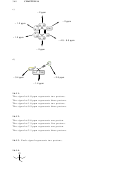 8
8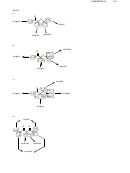 9
9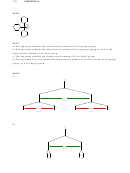 10
10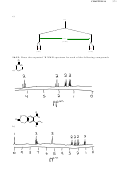 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19








