Gram/mole Conversions Worksheet With Answers
ADVERTISEMENT
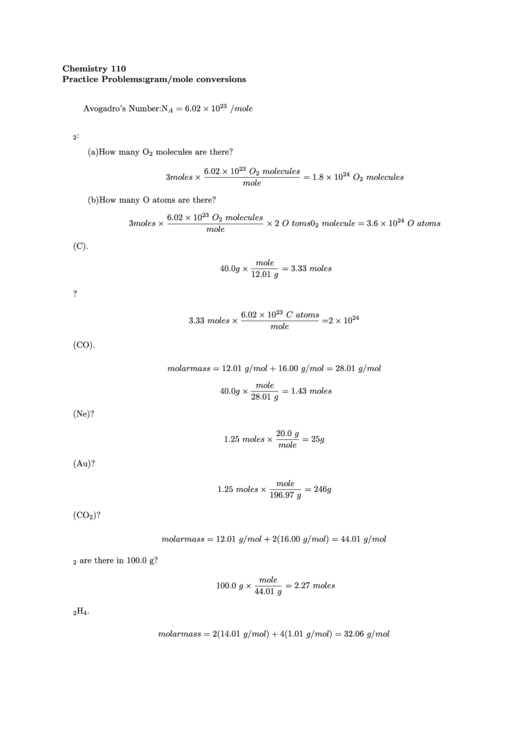
Chemistry 110
Practice Problems: gram/mole conversions
Avogadro’s Number: N
= 6.02
10
/mole
A
1. In 3 moles of O :
(a) How many O molecules are there?
6.02
10
O molecules
3moles
= 1.8
10
O molecules
mole
(b) How many O atoms are there?
6.02
10
O molecules
3moles
2 O toms0 molecule = 3.6
10
O atoms
mole
2. Calculate the number of moles in 40.0 g of carbon (C).
mole
40.0g
= 3.33 moles
12.01 g
3. How many carbon atoms are there in 40.0 g?
6.02
10
C atoms
3.33 moles
= 2
10
mole
4. Calculate the number of moles in 40.0 g of carbon monoxide (CO).
molar mass = 12.01 g/mol + 16.00 g/mol = 28.01 g/mol
mole
40.0g
= 1.43 moles
28.01 g
5. How many grams are in 1.25 moles of neon (Ne)?
20.0 g
1.25 moles
= 25 g
mole
6. How many grams are in 1.25 moles of gold (Au)?
mole
1.25 moles
= 246 g
196.97 g
7. What is the molar mass of carbon dioxide (CO )?
molar mass = 12.01 g/mol + 2(16.00 g/mol) = 44.01 g/mol
8. How many moles of CO are there in 100.0 g?
mole
100.0 g
= 2.27 moles
44.01 g
9. Calculate the molar mass of N H .
molar mass = 2(14.01 g/mol) + 4(1.01 g/mol) = 32.06 g/mol
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








