Periodic Table Review Worksheet With Answer Key Page 4
ADVERTISEMENT
1.
The following graphics represent the nuclei of atoms. Using a periodic table of elements, fill in the table.
Use a periodic table of the elements to answer these questions.
1. The following graphics represent the nuclei of atoms. Using a periodic table of elements, fill in the table.
1.
The following graphics represent the nuclei of atoms. Using a periodic table of elements, fill in the table.
What the nucleus
What is this
How many electrons does the
What is the mass
1. The following graphics represent the nuclei of atoms. Using a periodic table of elements, fill in the table.
looks like
element?
neutral atom have?
number?
What the nucleus
What is this
How many electrons does the
What is the mass
What the nucleus
What is this
How many electrons does the
What is the mass
Nucleus of Atom
Element
Electrons
Atomic Mass
looks like
element?
neutral atom have?
number?
looks like
element?
neutral atom have?
number?
What the nucleus
What is this
How many electrons does the
What is the mass
looks like
element?
neutral atom have?
number?
2.
Look at a periodic table. The atomic mass of hydrogen is 1.00794. Why is this number not rounded off to 1?
2. Look at a periodic table. The atomic mass of hydrogen is 1.00794. Why is this number not rounded off to 1?
2.
Look at a periodic table. The atomic mass of hydrogen is 1.00794. Why is this number not rounded off to 1?
Directions: label the trends of the periodic table by adding arrows and descriptions.
3.
How many protons and neutrons are in the nucleus of each isotope?
2. Look at a periodic table. The atomic mass of hydrogen is 1.00794. Why is this number not rounded off to 1
3. How many protons and neutrons are in the nucleus of each isotope?
3.
How many protons and neutrons are in the nucleus of each isotope?
a.
hydrogen-2 (atomic number = 1)
3. How many protons and neutrons are in the nucleus of each isotope?
a.
hydrogen-2 (atomic number = 1)
a.
hydrogen-2 (atomic number = 1)
b.
scandium-45 (atomic number = 21)
a.
hydrogen-2 (atomic number = 1)
b. scandium-45 (atomic number = 21)
b.
scandium-45 (atomic number = 21)
c.
aluminum-27 (atomic number = 13)
b. scandium-45 (atomic number = 21)
c.
aluminum-27 (atomic number = 13)
c.
aluminum-27 (atomic number = 13)
d.
uranium-235 (atomic number = 92)
c.
aluminum-27 (atomic number = 13)
d. uranium-235 (atomic number = 92)
d.
uranium-235 (atomic number = 92)
e.
carbon-12 (atomic number = 6)
d. uranium-235 (atomic number = 92)
e.
carbon-12 (atomic number = 6)
e.
carbon-12 (atomic number = 6)
4.
Although electrons have mass, they are not considered in determining the mass number or atomic mass of an
e.
carbon-12 (atomic number = 6)
atom. Why?
4. Although electrons have mass, they are not considered in determining the mass number or atomic mass of an
4.
Although electrons have mass, they are not considered in determining the mass number or atomic mass of an
atom. Why?
4. Although electrons have mass, they are not considered in determining the mass number or atomic mass of a
atom. Why?
5.
A hydrogen atom has one proton, two neutrons, and no electrons. Is this atom neutrally charged? Explain
atom. Why?
your answer.
5. A hydrogen atom has one proton, two neutrons, and no electrons. Is this atom neutrally charged? Explain
5.
A hydrogen atom has one proton, two neutrons, and no electrons. Is this atom neutrally charged? Explain
your answer.
5. A hydrogen atom has one proton, two neutrons, and no electrons. Is this atom neutrally charged? Explain
your answer.
6.
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. Given this information, how many
your answer.
electrons does it have? How many protons and neutrons does this atom have?
6. An atom of sodium-23 (atomic number = 11) has a positive charge of +1. Given this information, how many
6.
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. Given this information, how many
electrons does it have? How many protons and neutrons does this atom have?
6. An atom of sodium-23 (atomic number = 11) has a positive charge of +1. Given this information, how many
electrons does it have? How many protons and neutrons does this atom have?
electrons does it have? How many protons and neutrons does this atom have?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7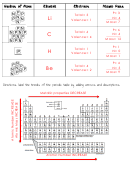 8
8