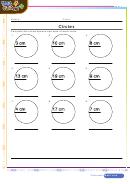
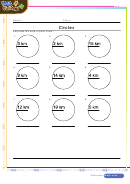
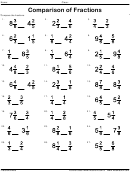Degree Of Unsaturation Worksheet With Answer Key Page 2
ADVERTISEMENT
Saturated vs. Unsaturated Molecules
In the lab,
saturation
may be thought of as the point when a solution cannot dissolve anymore of a substance added to it.
In terms of degrees of unsaturation, a molecule only containing single bonds with no rings is considered saturated.
chewiki_sat2
(3).bmp
chewiki_sat.bmp
1-methyoxypentane
CH
CH
CH
3
2
3
Unlike saturated molecules, unsaturated molecules contain double bond(s), triple bond(s) and/or ring(s).
chewiki_unsat1.bmp chewiki_unsat2.bmp
chewiki_unsat3.bmp
3-chloro-5-octyne
CH
CH=CHCH
3
3
Calculating Degrees of Unsaturation (DoU)
If the molecular formula is given, plug in the numbers into this formula:
\[ DoU= \dfrac{2C+2+N-X-H}{2} \]
• \(C\) is the number of carbons
• \(N\) is the number of nitrogens
• \(X\) is the number of halogens (F, Cl, Br, I)
• \(H\) is the number of hydrogens
As stated before, a saturated molecule contains only single bonds and no rings. Another way of interpreting this
is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. Thus,
the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As
an example, for the molecular formula C
H
the number of actual hydrogens needed for the compound to be saturated
3
4
is 8 [2C+2=(2x3)+2=8]. The compound needs 4 more hydrogens in order to be fully saturated (expected number of
hydrogens-observed number of hydrogens=8-4=4). Degrees of unsaturation is equal to 2, or half the number of hydrogens
the molecule needs to be classified as saturated. Hence, the DoB formula divides by 2. The formula subtracts the number
of X's because a halogen (X) replaces a hydrogen in a compound. For instance, in chloroethane, C
H
Cl, there is one
2
5
less hydrogen compared to ethane, C
H
.
2
6
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6