Kinetics/equilibrium Review Worksheet With Answers - Regents Chemistry Mr. Woods Page 4
ADVERTISEMENT
Kinetics/Equilibrium Review
20. Base your answer to the following question on the information below.
At 550°C, 1.00 mole of CO
(g) and 1.00 mole of H
(g) are placed in a 1.00-liter reaction vessel. The
2
2
substances react to form CO(g) and H
O(g). Changes in the concentrations of the reactants and the
2
concentrations of the products are shown in the graph below.
What can be concluded from the graph about the concentrations of the reactants and the concentrations of
the products between time t
and time t
?
1
2
21. Base your answer to the following question on the
information below.
The balanced equation below represents the
decomposition of potassium chlorate.
(s) ® 2KCl(s) + 3O
2KClO
(g)
3
2
State why the entropy of the reactant is less than the
entropy of the products.
Regents Chemistry
Mr. Woods
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4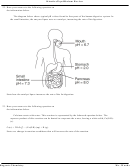 5
5 6
6








