Mini-Mock Unit 3 Aqa Gcse Chemistry Higher Tier Worksheet Page 16
ADVERTISEMENT
M14.
(a)
1213.8 to 1214.3
1) moles of N
=
= 35.7 mol
2
2) moles of NH
= 2 × (answer from (1)) = 71.4 mol
3
3) mass of NH
= (answer from 2) × 17 = 71.4 × 17 = 1214 g
3
3
or
•
28g of N
→ 34g of NH
2
3
•
1g of N
→
= 1.214g NH
2
3
•
1000 g of N
→ 1000 × 1.214= 1214g
2
or
•
1000 ×
1000 ×
(823.5g) 1000 ×
gains 1 mark if calculated correctly (1647.05g)
(b)
25 / 25.035
27.6 / 28
2
(c)
(i)
increase yield; reaction is exothermic
OR allow decreased yield because rate of reaction is slower /
fewer collisions
1
(ii)
increase yield
1
plus one from:
•
more (gaseous) reactant molecules than (gaseous)
product molecules (owtte)
accept greater volume on the left than the right owtte
•
increased rate of reaction / more collisions
1
Page 16
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5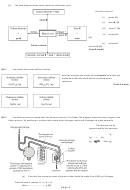 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18








