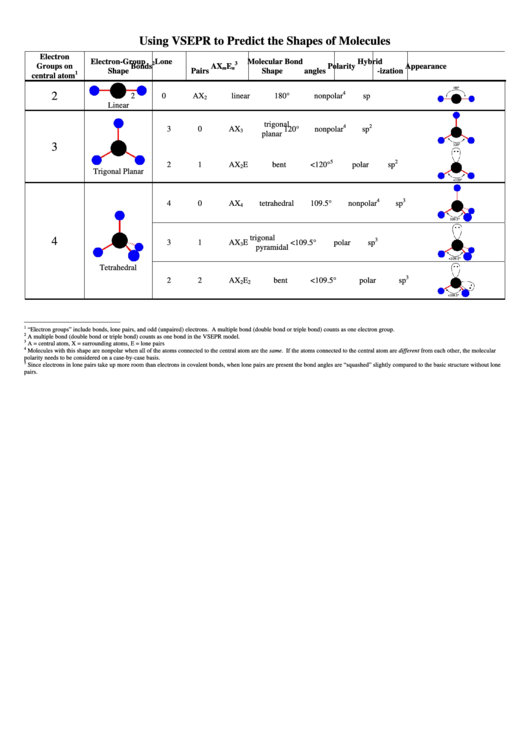
Vsepr Chart Using Vsepr To Predict The Shapes Of Molecules
ADVERTISEMENT
Using VSEPR to Predict the Shapes of Molecules
Electron
Electron-Group
Lone
Molecular
Bond
Hybrid
2
3
Groups on
Bonds
AX
E
Polarity
Appearance
m
n
Shape
Pairs
Shape
angles
-ization
1
central atom
180°
4
2
2
0
AX
linear
180°
nonpolar
sp
2
Linear
trigonal
4
2
3
0
AX
120°
nonpolar
sp
3
planar
3
120°
5
2
2
1
AX
E
bent
<120°
polar
sp
2
Trigonal Planar
<120°
4
3
4
0
AX
tetrahedral
109.5°
nonpolar
sp
4
109.5°
trigonal
4
3
3
1
AX
E
<109.5°
polar
sp
3
pyramidal
<109.5°
Tetrahedral
3
2
2
AX
E
bent
<109.5°
polar
sp
2
2
<109.5°
1
“Electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group.
2
A multiple bond (double bond or triple bond) counts as one bond in the VSEPR model.
3
A = central atom, X = surrounding atoms, E = lone pairs
4
Molecules with this shape are nonpolar when all of the atoms connected to the central atom are the same. If the atoms connected to the central atom are different from each other, the molecular
polarity needs to be considered on a case-by-case basis.
5
Since electrons in lone pairs take up more room than electrons in covalent bonds, when lone pairs are present the bond angles are “squashed” slightly compared to the basic structure without lone
pairs.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








