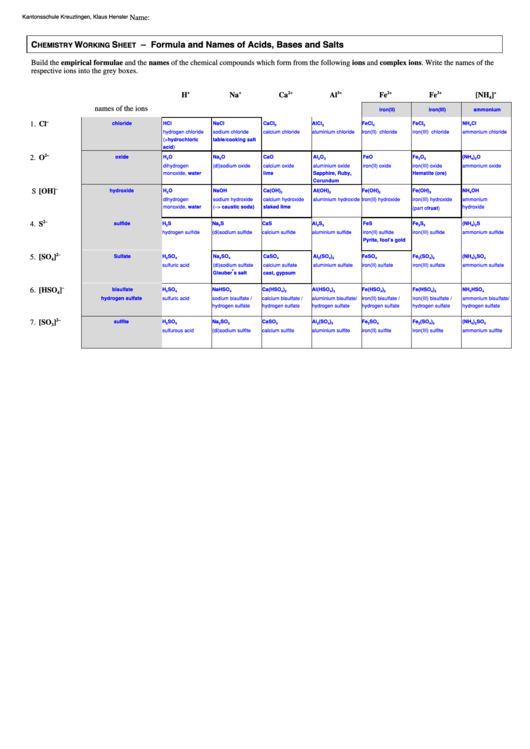
Chemistry Working Sheet - Formula And Names Of Acids, Bases And Salts
ADVERTISEMENT
Kantonsschule Kreuzlingen, Klaus Hensler
Name:
C
W
S
– Formula and Names of Acids, Bases and Salts
HEMISTRY
ORKING
HEET
Build the empirical formulae and the names of the chemical compounds which form from the following ions and complex ions. Write the names of the
respective ions into the grey boxes.
+
+
2+
3+
2+
3+
+
H
Na
Ca
Al
Fe
Fe
[NH
]
4
names of the ions
iron(II)
iron(III)
ammonium
–
chloride
HCl
NaCl
CaCl
AlCl
FeCl
FeCl
NH
Cl
1. Cl
2
3
2
3
4
hydrogen chloride
sodium chloride
calcium chloride
aluminium chloride
iron(II) chloride
iron(III) chloride
ammonium chloride
(> hydrochloric
table/cooking salt
acid)
2–
oxide
H
O
Na
O
CaO
Al
O
FeO
Fe
O
(NH
)
O
2. O
2
2
2
3
2
3
4
2
dihydrogen
(di)sodium oxide
calcium oxide
aluminium oxide
iron(II) oxide
iron(III) oxide
ammonium oxide
monoxide, water
lime
Sapphire, Ruby,
Hematite (ore)
Corundum
–
hydroxide
H
O
NaOH
Ca(OH)
Al(OH)
Fe(OH)
Fe(OH)
NH
OH
S [OH]
2
2
3
2
3
4
dihydrogen
sodium hydroxide
calcium hydroxide
aluminium hydroxide
iron(II) hydroxide
iron(III) hydroxide
ammonium
monoxide, water
(–> caustic soda)
slaked lime
hydroxide
(part of rust)
2–
sulfide
H
S
Na
S
CaS
Al
S
FeS
Fe
S
(NH
)
S
4. S
2
2
2
3
2
3
4
2
hydrogen sulfide
(di)sodium sulfide
calcium sulfide
aluminium sulfide
iron(II) sulfide
iron(III) sulfide
ammonium sulfide
Pyrite, fool’s gold
2–
Sulfate
H
SO
Na
SO
CaSO
Al
(SO
)
FeSO
Fe
(SO
)
(NH
)
SO
5. [SO
]
2
4
2
4
4
2
4
3
4
2
4
3
4
2
4
4
sulfuric acid
(di)sodium sulfate
calcium sulfate
aluminium sulfate
iron(II) sulfate
iron(III) sulfate
ammonium sulfate
Glauber’s salt
cast, gypsum
–
bisulfate
H
SO
NaHSO
Ca(HSO
)
Al(HSO
)
Fe(HSO
)
Fe(HSO
)
NH
HSO
6. [HSO
]
2
4
4
4
2
4
3
4
2
4
3
4
4
4
hydrogen sulfate
sulfuric acid
sodium bisulfate /
calcium bisulfate /
aluminium bisulfate/
iron(II) bisulfate /
iron(III) bisulfate /
ammonium bisulfate/
hydrogen sulfate
hydrogen sulfate
hydrogen sulfate
hydrogen sulfate
hydrogen sulfate
hydrogen sulfate
2–
sulfite
H
SO
Na
SO
CaSO
Al
(SO
)
Fe
SO
Fe
(SO
)
(NH
)
SO
7. [SO
]
2
3
2
3
3
2
4
3
2
4
2
4
3
4
2
3
3
sulfurous acid
(di)sodium sulfite
calcium sulfite
aluminium sulfite
iron(II) sulfite
iron(III) sulfite
ammonium sulfite
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








