Balancing Equations / Types Of Reaction Review
ADVERTISEMENT
Balancing Equations / Types of Reaction Review
Type SYN, DEC,SR,DR,CC, ICC
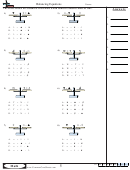
1. _1_S
+ _12__O
---> __8_SO
SYN
8
2
3
Sulfur reacts with oxygen to form sulfur trioxide
2. __2_HgO --->__2_Hg + __1_ O
DEC
2
Mercury (II) oxide decomposes to mercury and oxygen
3. __2_Na + __1_H
O ---> __2_NaOH + __1_H
SR
2
2
sodium reacts with water to form sodium hydroxide and hydrogen
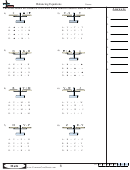
4. __2_H
PO
---> __1_H
P
O
+ __1_H
O
DEC
3
4
4
2
7
2
phosphoric acid decomposies into pyrophosphoric acid and water
5. __2_Al(OH)
+ __3_ H
SO
(aq ) ---> __1_Al
(SO
)
+__6_ H
O
DR
3
2
4
2
4
3
2
aluminium hydroxide reacts with sulfuric acid to from aluminium sulfate and water
6. __1_Fe
(SO
)
+ __2_KOH ---> __1_ K
SO
+ __2__Fe(OH)
DR
2
4
3
2
4
3
iron(III) sulfate reacts with potassium hydroxide to form potassium sulfate and iron(III) hydroxide
7. __2_C
H
O
+ _16__ O
---> __14_ CO
+ __6_H
O
CC
7
6
2
2
2
2
8. _2__Al + _3__ FeO ---> ___1 Al
O
+ __3_ Fe
SR
2
3
aluminium reacts with iron(II)oxide to form aluminium oxide and iron
9. _1__Fe
O
+ __3_ H
---> _2__ Fe + __3_ H
O
SR
2
3
2
2
iron(III)oxide reacts with hydrogen to form iron and water
10.__2_ K + _1__ Br
---> __2_ KBr
SYN
2
potassium reacts with bromine to from potassium bromide
11.__1_K
O + __1_H
O ---> __2_ KOH
SYN
2
2
potassium oxide reacts with water to form potassium hydroxide
12.__2_H
O
---> __2_ H
O + __1_ O
DEC
2
2
2
2
dihydrogen dioxide decomposes into water and oxygen
13.__1_SiO
+ __4_ HF(aq) ---> __1_ SiF
+ __2__ H
O
DR
2
4
2
silicon dioxide reacts with hydrofluoric acid to from silicon tetrafluoride and water
14.__2_H
AsO
---> __1_As
O
+ __3__ H
O
DEC
3
4
2
5
2
15. __1_ C
H
+ _3__ O
---> __1_ C + _2__ CO + __4_ H
O
ICC
3
8
2
2
propane reacts incompletely with oxygen to form carbon, carbon monoxide and water
16.__1__P
O
+ __6__H
O ---> __4__ H
PO
(aq)
SYN
4
10
2
3
4
tetraphosphorous pentaoxide reacts with water to from phosphoric acid
17.__4__Sb + _3__ O
---> _1__ Sb
O
SYN
2
4
6
antimony reacts with oxygen to form tetra antimony hexaoxide
18.__1_N
O
+ _1__ H
O ---> _2__ HNO
SYN
2
5
2
3
dinitrogen pentoxide reacts with water to form nitric acid
19. __1_Fe
O
+ __3_ CO ---> _2__ Fe + _3__ CO
2
3
2
iron (III) oxide reacts with carbon monoxide to form iron and carbon dioxide
20. _6__H
BO
---> __1_ H
B
O
+ __7_ H
O
DEC
3
3
4
6
11
2
21.__8_H
S + __8_Cl
---> __1_ S
+ ___16_ HCl
SR
2
2
8
dihydrogen sulfide reacts with chlorine to form sulfur and hydrogen chloride
22 __1_H
SO
(aq) + _2__KOH ---> __1_ K
SO
+ ___2_ H
O
DR
2
3
2
3
2
sulfurous acid reacts with potassium hydroxide to from potassium sulfite and water
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2