Atomic Structure Practice
ADVERTISEMENT
Name: ___________________________________ Period: _____ Date: _____________
Atomic Structure Practice
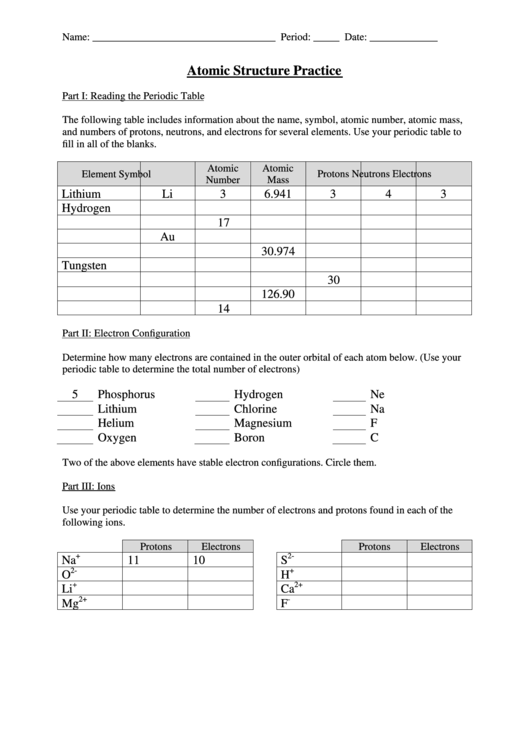
Part I: Reading the Periodic Table
The following table includes information about the name, symbol, atomic number, atomic mass,
and numbers of protons, neutrons, and electrons for several elements. Use your periodic table to
fill in all of the blanks.
Atomic
Atomic
Element
Symbol
Protons
Neutrons
Electrons
Number
Mass
Lithium
Li
3
6.941
3
4
3
Hydrogen
17
Au
30.974
Tungsten
30
126.90
14
Part II: Electron Configuration
Determine how many electrons are contained in the outer orbital of each atom below. (Use your
periodic table to determine the total number of electrons)
5
Phosphorus
Hydrogen
Ne
Lithium
Chlorine
Na
Helium
Magnesium
F
Oxygen
Boron
C
Two of the above elements have stable electron configurations. Circle them.
Part III: Ions
Use your periodic table to determine the number of electrons and protons found in each of the
following ions.
Protons
Electrons
Protons
Electrons
+
2-
Na
11
10
S
2-
+
O
H
+
2+
Li
Ca
2+
-
Mg
F
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1