Delocalized Electrons: Resonance Structures - Key Page 3
ADVERTISEMENT
CHEM 109A
CLAS
Delocalized Electrons: Resonance Structures - KEY
Combine most significant resonance structures to get the resonance hybrid (actual
structure of molecule).
Drawing Resonance Strucutures/Contributors:
See also Special Topic V: Drawing Resonance Contributors in Study Guide and
Solutions Manual of text.
Tail of a curved arrow shows where e- came from (bond or lone pair, never a +
charge or empty space).
Head of curved arrow shows where e- go to (point between atoms to form bond
or point to an atom to form lone pair, never into – charge and only very rarely into
empty space (EX. Diels-Alder reaction)).
Two rules/commandments
1. Do NOT break a single bond.
2. Do NOT exceed an octet for second-row elements (C, N, O, F) – can
have less than an octet (use formal charge) but NOT more – or 12
electrons for a third-row element (P, S, etc).
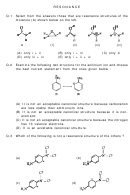
O
violates rule 2
R
OK
N
N
+
violates rule 1
Predicting Relative Stability of Resonance Structures/Contributors:
Factors that INCREASE Stability
Factors that DECREASE Stability
complete octets on all atoms
incomplete octets
no formal charge (FC)
separated charges/large FC/
like charges on adjacent atoms
more covalent bonds
fewer covalent bonds
negative FC on more EN atoms
negative FC on less EN atoms
positive FC on less EN atoms
positive FC on more EN atoms
Some examples comparing stabilities
-
O
O
HO
H
+HO
H
less stable
+
-
O
O
O
-
+
C
C
most stable
least stable
-
-
N
N
N
+N
N
N
+
+
H
C
H
C
3
3
less stable
Page 3 of 4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4