Chem 2 Ap Homework Atomic Orbitals With Answers Page 2
ADVERTISEMENT
2
HOMEWORK #7-4 ANSWER KEY
77
Explain what is meant by a noble gas core. Write the electron configuration of a xenon core.
A noble gas core is the electron configuration of the noble gas immediately preceding the atom of
interest and represents the inner electrons. For [Xe], the configuration would be
2
2
6
2
6
2
10
6
2
10
6
1s
2s
2p
3s
3p
4s
3d
4p
5s
4d
5p
.
80
The ground-state electron configurations listed here are incorrect. Explain what mistakes have
been made in each case and write the correct electron configurations.
2
2
4
2
3
Al: 1s
2s
2p
3s
3p
There are not enough electrons in the 2p subshell. (The 2p subshell holds six
electrons.) The number of electrons (13) is correct. The electron configuration should be
2
2
6
2
1
1s
2s
2p
3s
3p
. The configuration shown might be an excited state of an aluminum atom.
2
2
5
B: 1s
2s
2p
There are too many electrons. (Boron only has five electrons.) The electron
2
2
1
configuration should be 1s
2s
2p
. What would be the electric charge of a boron ion with the
electron arrangement given in the problem?
2
2
6
F: 1s
2s
2p
There are also too many electrons. (Fluorine only has nine electrons.) The
2
2
5
−
configuration shown is that of the F
ion. The correct electron configuration is 1s
2s
2p
.
83
Write the ground-state electron configurations for the following elements: B, V, Ni, As, I, Au
2
2
1
2
10
3
B: 1s
2s
2p
As: [Ar]4s
3d
4p
2
3
2
10
5
V: [Ar]4s
3d
I:
[Kr]5s
4d
5p
2
8
2
14
9
1
14
10
Ni: [Ar]4s
3d
Au: [Xe]6s
4f
5d
(actually 6s
4f
5d
due to an exception)
91
Use the Aufbau principle to obtain the ground-state electron configuration of selenium.
2
10
4
Se: [Ar]4s
3d
4p
124
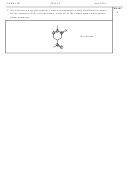
Shown below are portions of orbital diagrams representing
the ground-state electron configurations of certain elements.
Which of them violate the Pauli exclusion principle? Hund’s
rule?
The Pauli exclusion principle states that no two electrons in an
atom can have the same four quantum numbers. In other words,
only two electrons may exist in the same atomic orbital, and
these electrons must have opposite spins. (a) and (f) violate the
Pauli exclusion principle.
Hund’s rule states that the most stable arrangement of electrons in subshells is the one with the
greatest number of parallel spins. (b), (d), and (e) violate Hund’s rule. (In b and e, the single spin-
down electron is considered a higher-energy state.)
(c) is a valid configuration (no rule requires the first paired electron to be in the first p-orbital).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2