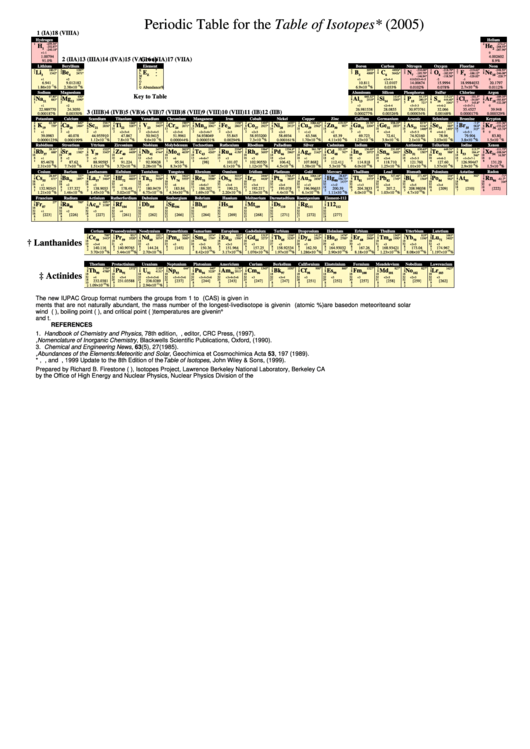
Periodic Table For The Table Of Isotopes
ADVERTISEMENT
Periodic Table for the Table of Isotopes* (2005)
1 (IA)
18 (VIIIA)
Hydrogen
Helium
1
-259.34°
2
-272.2°
H
He
-252.87°
-268.93°
1
2
-240.18°
-267.96°
+1-1
0
1.00794
4.002602
2 (IIA)
Group
13 (IIIA) 14 (IVA) 15 (VA) 16 (VIA) 17 (VIIA)
91.0%
8.9%
Lithium
Beryllium
Element
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Neon
2
180.5°
2
1287°
K
M.P.°
2
2075°
2
4492t°
2
-210.00°
2
-218.79°
2
-219.62°
2
-248.59°
Li
Be
E
B
C
N
O
F
Ne
1
1342°
2
2471°
L
B.P.°
3
4000°
4
3642s°
5
-195.79°
6
-182.95°
7
-188.12°
8
-246.08°
3
4
Z
5
6
7
8
9
10
M
C.P.°
-146.94°
-118.56°
-129.02°
-228.7°
±1±2±3+4+5
N
+1
+2
Ox.States
+3
+2+4-4
-2
-1
0
O
6.941
9.012182
At.Weight
10.811
12.0107
14.00674
15.9994
18.9984032
20.1797
P
-7
-9
-8
-6
1.86×10
%
2.38×10
%
Abundance%
6.9×10
%
0.033%
0.0102%
0.078%
2.7×10
%
0.0112%
Q
Sodium
Magnesium
Aluminum
Silicon
Phosphorus
Sulfur
Chlorine
Argon
Key to Table
2
97.80°
2
650°
2
660.32°
2
1414°
2
44.15°
2
115.21°
2
-101.5°
2
-189.35°
Na
Mg
Al
Si
P
S
Cl
Ar
8
8
8
8
8
8
8
8
883°
1090°
2519°
3265°
280.5°
444.60°
-34.04°
-185.85°
11
12
13
14
15
16
17
18
1
2
3
4
5
6
7
8
721°
1041°
143.8°
-122.28°
+1
+2
+3
+2+4-4
+3+5-3
+4+6-2
+1+5+7-1
0
22.989770
24.3050
26.981538
28.0855
30.973761
32.066
35.4527
39.948
3 (IIIB)
4 (IVB)
5 (VB)
6 (VIB) 7 (VIIB) 8 (VIII)
9 (VIII) 10 (VIII) 11 (IB)
12 (IIB)
0.000187%
0.00350%
0.000277%
0.00326%
0.000034%
0.00168%
0.000017%
0.000329%
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
Gallium
Germanium
Arsenic
Selenium
Bromine
Krypton
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
63.38°
842°
1541°
1668°
1910°
1907°
1246°
1538°
1495°
1455°
1084.62°
419.53°
29.76°
938.25°
817t°
221°
-7.2°
-157.36°
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
8
759°
8
1484°
8
2836°
8
3287°
8
3407°
8
2671°
8
2061°
8
2861°
8
2927°
8
2913°
8
2562°
8
907°
8
2204°
8
2833°
8
614s°
8
685°
8
58.8°
8
-153.22°
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
8
8
9
10
11
13
13
14
15
16
18
18
18
18
18
18
18
18
1400°
1493°
315°
-63.74°
1
2
2
2
2
1
2
2
2
2
1
2
3
4
5
6
7
8
+1
+2
+3
+2+3+4
+2+3+4+5
+2+3+6
+2+3+4+7
+2+3
+2+3
+2+3
+1+2
+2
+3
+2+4
+3+5-3
+4+6-2
+1+5-1
0
39.0983
40.078
44.955910
47.867
50.9415
51.9961
54.938049
55.845
58.933200
58.6934
63.546
65.39
69.723
72.61
74.92160
78.96
79.904
83.80
-7
-6
-7
-6
-6
-6
-7
-7
-8
-7
-8
-7
0.0000123%
0.000199%
1.12×10
%
7.8×10
%
9.6×10
%
0.000044%
0.000031%
0.00294%
7.3×10
%
0.000161%
1.70×10
%
4.11×10
%
1.23×10
%
3.9×10
%
2.1×10
%
2.03×10
%
3.8×10
%
1.5×10
%
Rubidium
Strontium
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
Silver
Cadmium
Indium
Tin
Antimony
Tellurium
Iodine
Xenon
2
39.31°
2
777°
2
1522°
2
1855°
2
2477°
2
2623°
2
2157°
2
2334°
2
1964°
2
1554.9°
2
961.78°
2
321.07°
2
156.60°
2
231.93°
2
630.63°
2
449.51°
2
113.7°
2
-111.75°
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
8
8
8
8
8
8
8
8
8
8
8
8
8
8
8
8
8
8
688°
1382°
3345°
4409°
4744°
4639°
4265°
4150°
3695°
2963°
2162°
767°
2072°
2602°
1587°
988°
184.4°
-108.04°
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
546°
18
16.58°
8
8
9
10
12
13
13
15
16
18
18
18
18
18
18
18
18
18
+1
+2
+3
+4
+3+5
+6
+4+6+7
+3
+3
+2+4
+1
+2
+3
+2+4
+3+5-3
+4+6-2
+1+5+7-1
0
1
2
2
2
1
1
2
1
1
0
1
2
3
4
5
6
7
8
85.4678
87.62
88.90585
91.224
92.90638
95.94
[98]
101.07
102.90550
106.42
107.8682
112.411
114.818
118.710
121.760
127.60
126.90447
131.29
-8
-8
-8
-8
-9
-9
-9
-9
-9
-9
-9
-10
-8
-9
-8
-9
-8
2.31×10
%
7.7×10
%
1.51×10
%
3.72×10
%
2.28×10
%
8.3×10
%
6.1×10
%
1.12×10
%
4.5×10
%
1.58×10
%
5.3×10
%
6.0×10
%
1.25×10
%
1.01×10
%
1.57×10
%
2.9×10
%
1.5×10
%
Cesium
Barium
Lanthanum
Hafnium
Tantalum
Tungsten
Rhenium
Osmium
Iridium
Platinum
Gold
Mercury
Thallium
Lead
Bismuth
Polonium
Astatine
Radon
2
28.44°
2
727°
2
918°
2
2233°
2
3017°
2
3422°
2
3186°
2
3033°
2
2446°
2
1768.4°
2
1064.18°
2
-38.83°
2
304°
2
327.46°
2
271.40°
2
254°
2
302°
2
-71°
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
†
8
671°
8
1897°
8
3464°
8
4603°
8
5458°
8
5555°
8
5596°
8
5012°
8
4428°
8
3825°
8
2856°
8
356.73°
8
1473°
8
1749°
8
1564°
8
962°
8
8
-61.7°
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
1477°
104°
18
18
18
32
32
32
32
32
32
32
32
32
32
32
32
32
32
32
+1
+2
+3
+4
+5
+6
+4+6+7
+3+4
+3+4
+2+4
+1+3
+1+2
+1+3
+2+4
+3+5
+2+4
0
8
8
9
10
11
12
13
14
15
16
18
18
18
18
18
18
18
18
132.90545
137.327
138.9055
178.49
180.9479
183.84
186.207
190.23
192.217
195.078
196.96655
200.59
204.3833
207.2
208.98038
[209]
[210]
[222]
1
2
2
2
2
2
2
2
2
2
1
2
3
4
5
6
7
8
-9
-8
-9
-10
-11
-10
-10
-9
-9
-9
-10
-9
-10
-8
-10
1.21×10
%
1.46×10
%
1.45×10
%
5.02×10
%
6.75×10
%
4.34×10
%
1.69×10
%
2.20×10
%
2.16×10
%
4.4×10
%
6.1×10
%
1.11×10
%
6.0×10
%
1.03×10
%
4.7×10
%
Francium
Radium
Actinium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Meitnerium
Darmstadtium
Roentgenium
Element-112
2
27°
2
700°
2
1051°
2
2
2
2
2
2
2
2
2
Fr
Ra
Ac
‡
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
112
8
8
8
3198°
8
8
8
8
8
8
8
8
8
87
88
89
104
105
106
107
108
109
110
111
112
18
18
18
18
18
18
18
18
18
18
18
18
32
32
32
32
32
32
32
32
32
32
32
32
+1
+2
+3
+4
18
18
18
32
32
32
32
32
32
32
32
32
[223]
[226]
[227]
[261]
[262]
[266]
[264]
[269]
[268]
[271]
[272]
[277]
8
8
9
10
11
12
13
14
15
16
17
18
1
2
2
2
2
2
2
2
2
2
2
2
Cerium
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
Ytterbium
Lutetium
2
798°
2
931°
2
1021°
2
1042°
2
1074°
2
822°
2
1313°
2
1356°
2
1412°
2
1474°
2
1529°
2
1545°
2
819°
2
1663°
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
8
8
8
8
8
8
8
8
8
8
8
8
8
8
3443°
3520°
3074°
3000°
1794°
1596°
3273°
3230°
2567°
2700°
2868°
1950°
1196°
3402°
58
59
60
61
62
63
64
65
66
67
68
69
70
71
† Lanthanides
18
18
18
18
18
18
18
18
18
18
18
18
18
18
19
21
22
23
24
25
25
27
28
29
30
31
32
32
+3+4
+3
+3
+3
+2+3
+2+3
+3
+3
+3
+3
+3
+3
+2+3
+3
9
8
8
8
8
8
9
8
8
8
8
8
8
9
140.116
140.90765
144.24
[145]
150.36
151.964
157.25
158.92534
162.50
164.93032
167.26
168.93421
173.04
174.967
2
2
2
2
2
2
2
2
2
2
2
2
2
2
-9
-10
-9
-10
-10
-9
-10
-9
-10
-10
-10
-10
-10
3.70×10
%
5.44×10
%
2.70×10
%
8.42×10
%
3.17×10
%
1.076×10
%
1.97×10
%
1.286×10
%
2.90×10
%
8.18×10
%
1.23×10
%
8.08×10
%
1.197×10
%
Thorium
Protactinium
Uranium
Neptunium
Plutonium
Americium
Curium
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
2
1750°
2
1572°
2
1135°
2
644°
2
640°
2
1176°
2
1345°
2
1050°
2
900°
2
860°
2
1527°
2
827°
2
827°
2
1627°
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
8
8
8
8
8
8
8
8
8
8
8
8
8
8
4788°
4131°
3228°
2011°
90
91
92
93
94
95
96
97
98
99
100
101
102
103
‡ Actinides
18
18
18
18
18
18
18
18
18
18
18
18
18
18
32
32
32
32
32
32
32
32
32
32
32
32
32
32
+4
+5+4
+3+4+5+6
+3+4+5+6
+3+4+5+6
+3+4+5+6
+3
+3+4
+3
+3
+3
+2+3
+2+3
+3
18
20
21
22
24
25
25
27
28
29
30
31
32
32
232.0381
231.03588
238.0289
[237]
[244]
[243]
[247]
[247]
[251]
[252]
[257]
[258]
[259]
[262]
10
9
9
9
8
8
9
8
8
8
8
8
8
9
-10
-11
1.09×10
%
2.94×10
%
2
2
2
2
2
2
2
2
2
2
2
2
2
2
The new IUPAC Group format numbers the groups from 1 to 18. The numbering system used by the Chemical Abstracts Service (CAS) is given in parentheses. For ele-
ments that are not naturally abundant, the mass number of the longest-lived isotope is given in brackets. The abundances (atomic %) are based on meteorite and solar
wind data. The melting point (M.P.), boiling point (B.P.), and critical point (C.P.)temperatures are given in °Celsius. Sublimation and critical temperatures are indicated by s
and t.
REFERENCES
1. Handbook of Chemistry and Physics, 78th edition, D.R. Lide, editor, CRC Press, (1997).
2. G.J. Leigh, Nomenclature of Inorganic Chemistry, Blackwells Scientific Publications, Oxford, (1990).
3. Chemical and Engineering News, 63(5), 27(1985).
4. E. Anders and N. Grevesse, Abundances of the Elements: Meteoritic and Solar, Geochimica et Cosmochimica Acta 53, 197 (1989).
* R.B. Firestone, C.M. Baglin, and S.Y.F. Chu, 1999 Update to the 8th Edition of the Table of Isotopes, John Wiley & Sons, (1999).
Prepared by Richard B. Firestone (rbf@lbl.gov), Isotopes Project, Lawrence Berkeley National Laboratory, Berkeley CA 94720. This work was supported
by the Office of High Energy and Nuclear Physics, Nuclear Physics Division of the U.S. Department of Energy under contract DE-AC03-76SF00098.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








