Chemical Reactions - The Science Spot
ADVERTISEMENT
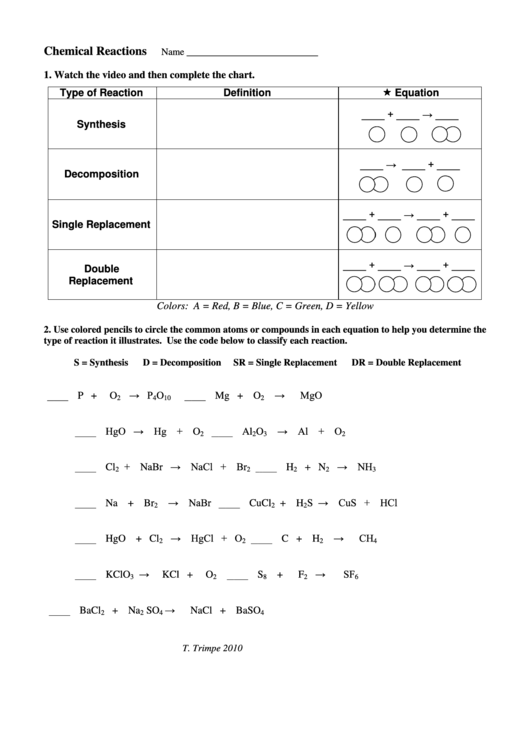
Chemical Reactions
Name ___________________________
1. Watch the video and then complete the chart.
Equation
Type of Reaction
Definition
____ + ____ → ____
Synthesis
____ → ____ + ____
Decomposition
____ + ____ → ____ + ____
Single Replacement
____ + ____ → ____ + ____
Double
Replacement
Colors: A = Red, B = Blue, C = Green, D = Yellow
2. Use colored pencils to circle the common atoms or compounds in each equation to help you determine the
type of reaction it illustrates. Use the code below to classify each reaction.
S = Synthesis
D = Decomposition
SR = Single Replacement
DR = Double Replacement
→ P
→
MgO
P +
O
O
Mg + O
______
2
4
10
______
2
HgO → Hg +
→ Al + O
O
Al
O
______
2
______
2
3
2
+ NaBr → NaCl + Br
→ NH
Cl
H
+ N
______
2
2
______
2
2
3
→ NaBr
S → CuS + HCl
Na + Br
CuCl
+ H
______
2
______
2
2
→ HgCl + O
→
CH
HgO + Cl
C + H
______
2
2
______
2
4
→
→
SF
KClO
KCl +
O
S
+
F
______
3
2
______
8
2
6
→
BaCl
+ Na
SO
NaCl + BaSO
______
2
2
4
4
T. Trimpe 2010
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








