Periodic Table Basics Class Work Page 2
ADVERTISEMENT
Name:__________________________ Period:_____ Date:________________
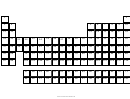
7. Elements are organized into families according to their physical and chemical properties.
Elements in the same family share similar chemical bonding properties.
Write the family names in the correct column on the chart below (See example). Then color
the columns according to the information below.
(Green) Alkali Metals have 1 valence electron
(Blue) Alkaline Earth Metals have 2 valence electrons.
(Pink) Boron Family has 3 valence electrons.
(Yellow) Carbon Family has 4 valence electrons.
(Brown) Nitrogen Family has 5 valence electrons.
(Red) Oxygen Family has 6 valence electrons.
(Orange) Halogens have 7 valence electrons.
(Light Blue) Noble Gases have 8 valence electrons.
(Light Green) Transition Metals have various valence electrons. They are in the "B" area
(3B, 4B, 5B, 6B, 7B, 8B, 1B, and 2B).
(Purple) Lanthanides have various valence electrons (elements 57-71).
(Black) Actinides have various valence electrons (elements 89-103).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2