Atomic Orbital Ionization Energies, Ev Chart
ADVERTISEMENT
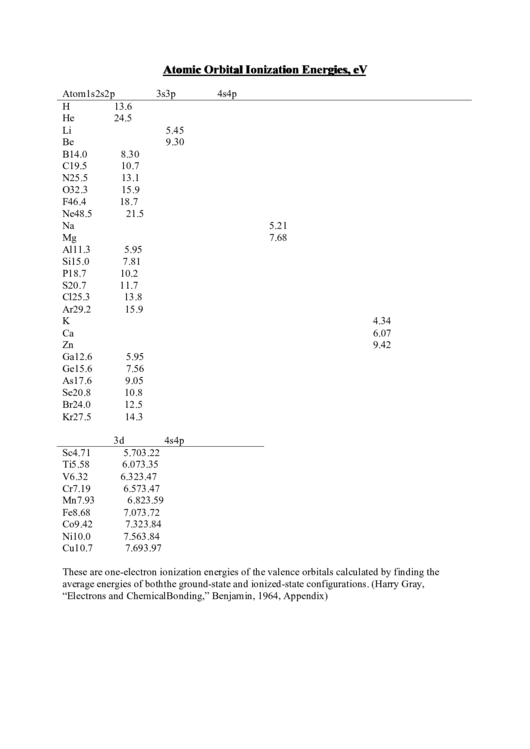
Atomic Orbital Ionization Energies, eV
Atom
1s
2s
2p
3s
3p
4s
4p
H
13.6
He
24.5
Li
5.45
Be
9.30
B
14.0
8.30
C
19.5
10.7
N
25.5
13.1
O
32.3
15.9
F
46.4
18.7
Ne
48.5
21.5
Na
5.21
Mg
7.68
Al
11.3
5.95
Si
15.0
7.81
P
18.7
10.2
S
20.7
11.7
Cl
25.3
13.8
Ar
29.2
15.9
K
4.34
Ca
6.07
Zn
9.42
Ga
12.6
5.95
Ge
15.6
7.56
As
17.6
9.05
Se
20.8
10.8
Br
24.0
12.5
Kr
27.5
14.3
3d
4s
4p
Sc
4.71
5.70
3.22
Ti
5.58
6.07
3.35
V
6.32
6.32
3.47
Cr
7.19
6.57
3.47
Mn
7.93
6.82
3.59
Fe
8.68
7.07
3.72
Co
9.42
7.32
3.84
Ni
10.0
7.56
3.84
Cu
10.7
7.69
3.97
These are one-electron ionization energies of the valence orbitals calculated by finding the
average energies of both the ground-state and ionized-state configurations. (Harry Gray,
“Electrons and Chemical Bonding,” Benjamin, 1964, Appendix)
ADVERTISEMENT
0 votes
 1
1








