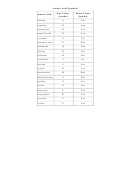
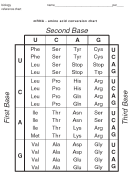
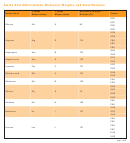
Amino Acid Pka Chart
ADVERTISEMENT
CHEM 108B, LECTURE 16
What is a pKa?
Titration of Phosphoric Acid (pKa
2.1; pKa
7.2; pKa
12.3)
1
2
3
What’s the charge/dominant ionic phosphate species at pH 1? pH 5? pH 7.4? pH 13?
Amino Acids – which ones look good to you?
R
R
R
R
+
+
-
-
CO
H
CO
H
N
H
N
H
N
CO
H
N
CO
H
3
2
3
2
2
2
2
2
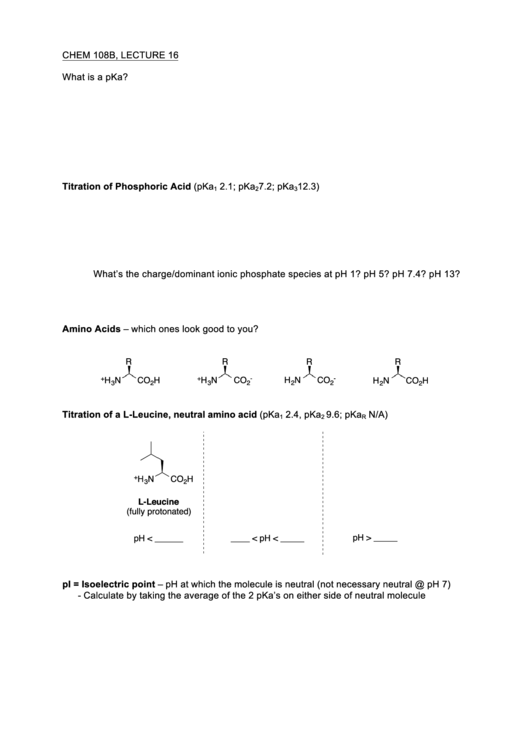
Titration of a L-Leucine, neutral amino acid (pKa
2.4, pKa
9.6; pKa
N/A)
1
2
R
+
H
N
CO
H
3
2
L-Leucine
(fully protonated)
pH > _____
pH < ______
____ < pH < _____
pI = Isoelectric point – pH at which the molecule is neutral (not necessary neutral @ pH 7)
-
Calculate by taking the average of the 2 pKa’s on either side of neutral molecule
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3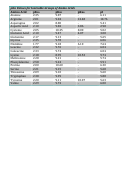 4
4