Midterm Chemistry Test Page 2
ADVERTISEMENT
6. (3) Assign symmetry point groups to the molecules from question 5.
2–
[BeCl
F
]
Point group:
2
2
̶
[ClO
]
Point group:
2
BrF
Point group:
5
7. (5) Assign a symmetry point group to each molecule in the series:
+
–
PClBrF
PF
[PF
]
PF
[PF
]
3
4
5
6
Point group:
_____
____
_____
_____
______
–
8. (a) (5) Prepare a qualitative molecular orbital diagram for the Be
ion (use the space provided below on
2
, σ
, π etc.).
this page). Name all molecular orbitals (σ
1
2
(b) (2) Calculate the bond order and the number of unpaired electrons in this ion.
(c) (1) Decide whether the molecule of Be
exists? If you say ‘yes’, should this compound have a sorter or
2
–
longer Be–Be bond compared to that in the Be
ion?
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2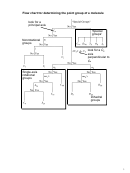 3
3








