Nmr Solvent Chart - Emery Pharma
ADVERTISEMENT
31175_CIL NMR Chart R2-orange
5/27/10
1:21 PM
Page 1
Cambridge Isotope Laboratories, Inc.
RESEARCH PRODUCTS
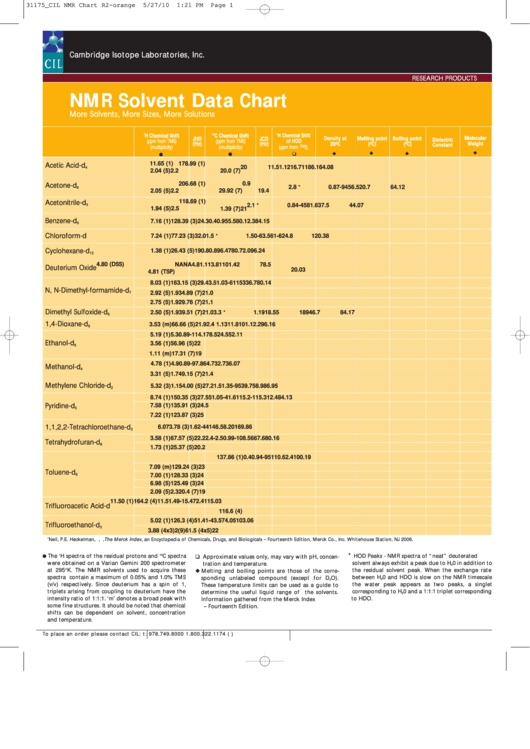
NMR Solvent Data Chart
More Solvents, More Sizes, More Solutions
1
H Chemical Shift
13
C Chemical Shift
1
H Chemical Shift
JHD
JCD
Density at
Melting point
Boiling point
Molecular
Dielectric
(ppm from TMS)
(ppm from TMS)
of HOD
(Hz)
(Hz)
20ºC
(ºC)
(ºC)
Weight
Constant
(multiplicity)
(multiplicity)
(ppm from TMS)
11.65 (1)
178.99 (1)
Acetic Acid-d
20
11.5
1.12
16.7
118
6.1
64.08
4
2.04 (5)
2.2
20.0 (7)
206.68 (1)
0.9
Acetone-d
2.8
*
0.87
-94
56.5
20.7
64.12
6
2.05 (5)
2.2
29.92 (7)
19.4
118.69 (1)
Acetonitrile-d
2.1
*
0.84
-45
81.6
37.5
44.07
3
1.94 (5)
2.5
1.39 (7)
21
Benzene-d
7.16 (1)
128.39 (3)
24.3
0.4
0.95
5.5
80.1
2.3
84.15
6
Chloroform-d
7.24 (1)
77.23 (3)
32.0
1.5
*
1.50
-63.5
61-62
4.8
120.38
Cyclohexane-d
1.38 (1)
26.43 (5)
19
0.8
0.89
6.47
80.7
2.0
96.24
12
4.80 (DSS)
NA
NA
4.8
1.11
3.81
101.42
78.5
Deuterium Oxide
20.03
4.81 (TSP)
8.03 (1)
163.15 (3)
29.4
3.5
1.03
-61
153
36.7
80.14
N, N-Dimethyl-formamide-d
2.92 (5)
1.9
34.89 (7)
21.0
7
2.75 (5)
1.9
29.76 (7)
21.1
Dimethyl Sulfoxide-d
2.50 (5)
1.9
39.51 (7)
21.0
3.3
1.19
18.55
189
46.7
84.17
*
6
1,4-Dioxane-d
3.53 (m)
66.66 (5)
21.9
2.4
1.13
11.8
101.1
2.2
96.16
8
5.19 (1)
5.3
0.89
-114.1
78.5
24.5
52.11
Ethanol-d
3.56 (1)
56.96 (5)
22
6
1.11 (m)
17.31 (7)
19
4.78 (1)
4.9
0.89
-97.8
64.7
32.7
36.07
Methanol-d
4
3.31 (5)
1.7
49.15 (7)
21.4
Methylene Chloride-d
5.32 (3)
1.1
54.00 (5)
27.2
1.5
1.35
-95
39.75
8.9
86.95
2
8.74 (1)
150.35 (3)
27.5
5
1.05
-41.6
115.2-115.3
12.4
84.13
Pyridine-d
7.58 (1)
135.91 (3)
24.5
5
7.22 (1)
123.87 (3)
25
1,1,2,2-Tetrachloroethane-d
6.0
73.78 (3)
1.62
-44
146.5
8.20
169.86
2
3.58 (1)
67.57 (5)
22.2
2.4-2.5
0.99
-108.5
66
7.6
80.16
Tetrahydrofuran-d
8
1.73 (1)
25.37 (5)
20.2
137.86 (1)
0.4
0.94
-95
110.6
2.4
100.19
7.09 (m)
129.24 (3)
23
Toluene-d
8
7.00 (1)
128.33 (3)
24
6.98 (5)
125.49 (3)
24
2.09 (5)
2.3
20.4 (7)
19
11.50 (1)
164.2 (4)
11.5
1.49
-15.4
72.4
115.03
Trifluoroacetic Acid-d
116.6 (4)
5.02 (1)
126.3 (4)
5
1.41
-43.5
74.05
103.06
Trifluoroethanol-d
3
3.88 (4x3)
2(9)
61.5 (4x5)
22
M.J. O’Neil, P.E. Heckelman, C.B. Koch, K.J. Roman, The Merck Index, an Encyclopedia of Chemicals, Drugs, and Biologicals – Fourteenth Edition, Merck Co., Inc. Whitehouse Station, NJ 2006.
*
The
H spectra of the residual protons and
C spectra
HOD Peaks - NMR spectra of “neat” deuterated
1
13
Approximate values only, may vary with pH, concen-
were obtained on a Varian Gemini 200 spectrometer
solvent always exhibit a peak due to H
0 in addition to
tration and temperature.
2
at 295°K. The NMR solvents used to acquire these
Melting and boiling points are those of the corre-
the residual solvent peak. When the exchange rate
spectra contain a maximum of 0.05% and 1.0% TMS
sponding unlabeled compound (except for D
O).
between H
0 and HDO is slow on the NMR timescale
2
2
(v/v) respectively. Since deuterium has a spin of 1,
the water peak appears as two peaks, a singlet
These temperature limits can be used as a guide to
triplets arising from coupling to deuterium have the
corresponding to H
0 and a 1:1:1 triplet corresponding
determine the useful liquid range of the solvents.
2
intensity ratio of 1:1:1. ‘m’ denotes a broad peak with
Information gathered from the Merck Index
to HDO.
some fine structures. It should be noted that chemical
– Fourteenth Edition.
shifts can be dependent on solvent, concentration
and temperature.
To place an order please contact CIL:
t: 978.749.8000
1.800.322.1174 (N.America)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1