Chemistry Worksheet - Multiple Choice
ADVERTISEMENT
Chapter 3
Name___________________________________
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
1) Water is able to form hydrogen bonds because
1)
A) each of the hydrogen atoms in a water molecule is weakly negative in charge.
B) the bonds that hold together the atoms in a water molecule are polar covalent bonds.
C) oxygen has a valence of 2.
D) the water molecule is shaped like a tetrahedron.
E) the oxygen atom in a water molecule has a weak positive charge.
2) Carbon dioxide (CO 2 ) is readily soluble in water, according to the equation CO 2 + H 2 O
H 2 CO 3 .
2)
Carbonic acid (H 2 CO 3 ) is a weak acid. Respiring cells release CO 2 . What prediction can we make
about the pH of blood as that blood first comes in contact with respiring cells?
A) Blood pH will increase slightly.
B) Blood pH will first decrease, then increase sharply as CO 2 combines with hemoglobin.
C) Blood pH will first increase, then decrease as CO 2 combines with hemoglobin.
D) Blood pH will decrease slightly.
E) Blood pH will remain unchanged.
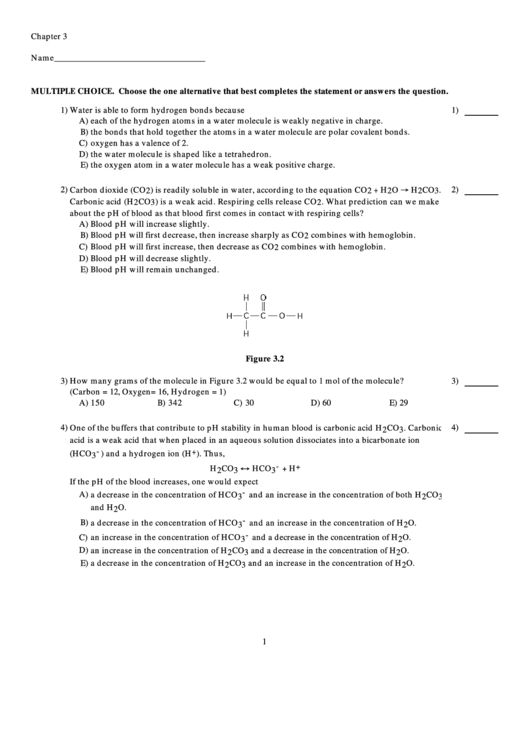
Figure 3.2
3) How many grams of the molecule in Figure 3.2 would be equal to 1 mol of the molecule?
3)
(Carbon = 12, Oxygen = 16, Hydrogen = 1)
A) 150
B) 342
C) 30
D) 60
E) 29
4) One of the buffers that contribute to pH stability in human blood is carbonic acid H 2 CO 3 . Carbonic
4)
acid is a weak acid that when placed in an aqueous solution dissociates into a bicarbonate ion
(HCO 3 - ) and a hydrogen ion (H + ). Thus,
HCO 3 - + H +
H 2 CO 3
If the pH of the blood increases, one would expect
A) a decrease in the concentration of HCO 3 - and an increase in the concentration of both H 2 CO 3
and H 2 O.
B) a decrease in the concentration of HCO 3 - and an increase in the concentration of H 2 O.
C) an increase in the concentration of HCO 3 - and a decrease in the concentration of H 2 O.
D) an increase in the concentration of H 2 CO 3 and a decrease in the concentration of H 2 O.
E) a decrease in the concentration of H 2 CO 3 and an increase in the concentration of H 2 O.
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








