Chemistry Worksheet
ADVERTISEMENT
CHE107-003
Practice Exam 3 Key
1. If 325 g of water at 4.2 °C absorbs 12.28 kJ, what is the final temperature
of the water? The specific heat of water is 4.184 J/g* °C.
13.2 °C
Δ
Use q = ms
t (keep track of units!)
2. A 0.1326 g sample of magnesium was burned in a constant-volume
(bomb) calorimeter. The total heat capacity (mass times specific heat) of
the calorimeter was 5,760 J/ C. If the temperature rise of the calorimeter
was 0.570°C, calculate the enthalpy of combustion of magnesium.
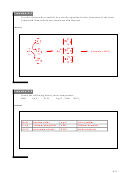
ΔH
Mg
+ ½O
→ MgO
= ?
(s)
2(g)
(s)
rxn
-602 kJ/mol
qcal = -qrxn
Δ
qcal = ms
t
3. When 0.560 g of Na
reacts with excess F
to form NaF
, 13.8 kJ of
(s)
2(g)
(s)
heat is evolved. What is the standard enthalpy of formation (ΔH°
) of
f
? ΔH°
) = ΔH°
NaF
(F
(Na
) = 0.
(s)
f
2(g)
f
(s)
-570 kJ/mol
Write the balanced reaction, use the direct method.
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4