Chemistry: States Of Matter Cheat Sheet
ADVERTISEMENT
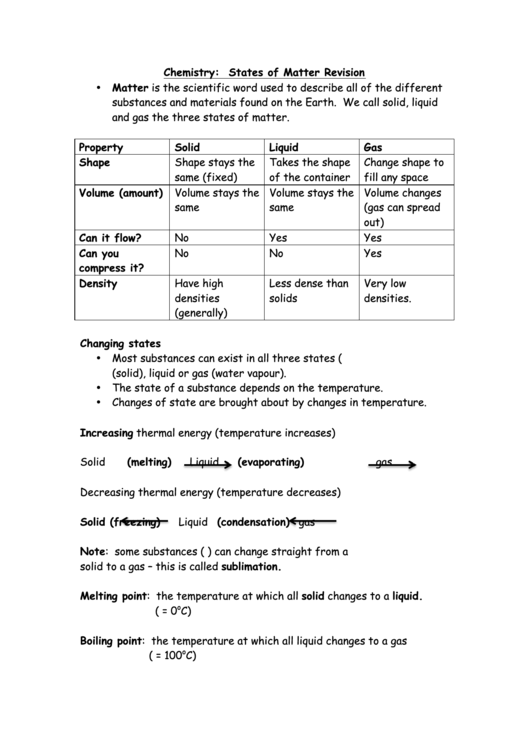
Chemistry: States of Matter Revision
• Matter is the scientific word used to describe all of the different
substances and materials found on the Earth. We call solid, liquid
and gas the three states of matter.
Property
Solid
Liquid
Gas
Shape
Shape stays the
Takes the shape
Change shape to
same (fixed)
of the container
fill any space
Volume (amount) Volume stays the
Volume stays the
Volume changes
same
same
(gas can spread
out)
Can it flow?
No
Yes
Yes
Can you
No
No
Yes
compress it?
Density
Have high
Less dense than
Very low
densities
solids
densities.
(generally)
Changing states
• Most substances can exist in all three states (e.g. water can be ice
(solid), liquid or gas (water vapour).
• The state of a substance depends on the temperature.
• Changes of state are brought about by changes in temperature.
Increasing thermal energy (temperature increases)
Solid
(melting)
Liquid
(evaporating)
gas
Decreasing thermal energy (temperature decreases)
Solid
(freezing)
Liquid
(condensation) gas
Note: some substances (e.g. Carbon dioxide) can change straight from a
solid to a gas – this is called sublimation.
Melting point: the temperature at which all solid changes to a liquid.
o
(e.g. water = 0
C)
Boiling point: the temperature at which all liquid changes to a gas
o
(e.g. water = 100
C)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2