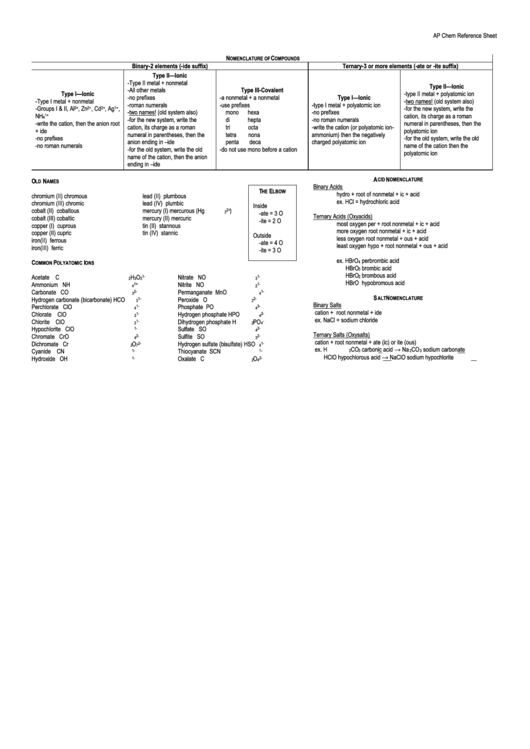
Ap Chem Reference Sheet
ADVERTISEMENT
AP Chem Reference Sheet
N
C
OMENCLATURE OF
OMPOUNDS
Binary-2 elements (-ide suffix)
Ternary-3 or more elements (-ate or -ite suffix)
Type II—Ionic
-Type II metal + nonmetal
Type II—Ionic
-All other metals
Type III-Covalent
Type I—Ionic
-type II metal + polyatomic ion
-no prefixes
-a nonmetal + a nonmetal
Type I—Ionic
-Type I metal + nonmetal
-two names! (old system also)
-roman numerals
-use prefixes
-type I metal + polyatomic ion
-Groups I & II, Al
3+
, Zn
2+
, Cd
2+
, Ag
1+
,
-for the new system, write the
-two names! (old system also)
mono
hexa
-no prefixes
NH
4 1+
cation, its charge as a roman
-for the new system, write the
di
hepta
-no roman numerals
-write the cation, then the anion root
numeral in parentheses, then the
cation, its charge as a roman
tri
octa
-write the cation (or polyatomic ion-
+ ide
polyatomic ion
numeral in parentheses, then the
tetra
nona
ammonium) then the negatively
-no prefixes
-for the old system, write the old
anion ending in –ide
penta
deca
charged polyatomic ion
-no roman numerals
name of the cation then the
-for the old system, write the old
-do not use mono before a cation
polyatomic ion
name of the cation, then the anion
ending in –ide
A
N
CID
OMENCLATURE
O
N
LD
AMES
Binary Acids
T
E
HE
LBOW
hydro + root of nonmetal + ic + acid
chromium (II)
chromous
lead (II)
plumbous
ex. HCl = hydrochloric acid
chromium (III)
chromic
lead (IV)
plumbic
Inside
cobalt (II)
cobaltous
mercury (I)
mercurous (Hg
2 2+
)
-ate = 3 O
Ternary Acids (Oxyacids)
cobalt (III)
cobaltic
mercury (II)
mercuric
-ite = 2 O
most oxygen
per + root nonmetal + ic + acid
copper (I)
cuprous
tin (II)
stannous
more oxygen
root nonmetal + ic + acid
copper (II)
cupric
tin (IV)
stannic
Outside
less oxygen
root nonmetal + ous + acid
iron(II)
ferrous
-ate = 4 O
least oxygen
hypo + root nonmetal + ous + acid
iron(III)
ferric
-ite = 3 O
ex. HBrO
perbrombic acid
C
P
I
4
OMMON
OLYATOMIC
ONS
HBrO
brombic acid
3
HBrO
brombous acid
Acetate
C
H
O
2 1-
Nitrate
NO
3 1-
2
2
3
HBrO
hypobromous acid
Ammonium
NH
4 1+
Nitrite
NO
2 1-
Carbonate
CO
3 2-
Permanganate
MnO
4 1-
S
N
ALT
OMENCLATURE
Hydrogen carbonate (bicarbonate)
HCO
3 1-
Peroxide
O
2 2-
Binary Salts
Perchlorate
ClO
4 1-
Phosphate
PO
4 3-
cation + root nonmetal + ide
Chlorate
ClO
3 1-
Hydrogen phosphate
HPO
4 2-
ex. NaCl = sodium chloride
Chlorite
ClO
2 1-
Dihydrogen phosphate
H
PO
4 -
2
Hypochlorite
ClO
Sulfate
SO
1-
4 2-
Ternary Salts (Oxysalts)
Chromate
CrO
4 2-
Sulfite
SO
3 2-
cation + root nonmetal + ate (ic) or ite (ous)
Dichromate
Cr
O
7 2-
Hydrogen sulfate (bisulfate) HSO
4 1-
2
ex. H
CO
carbonic acid → Na
CO
sodium carbonate
Cyanide
CN
1-
Thiocyanate
SCN
1-
2
3
2
3
HClO hypochlorous acid → NaClO sodium hypochlorite
Hydroxide
OH
1-
Oxalate
C
O
4 2-
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








