Chemistry Lesson Plan Template Page 17
ADVERTISEMENT
Notes
Representative elements
are elements in group 1A through 8A.
u
Valence electrons
are the electrons in the highest occupied energy level
u
of an element.
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
2,8,8,2
The Group Number tells the number of valence electrons.
u
Electron dot structures show the valence electrons of an element as
u
dots.
Be
H
C
B
Octet
Rule: ALL Atoms desire to achieve the stable noble gas
u
2
6
configuration (ns
np
) when they form compounds. This means having
8e
-
in their outermost energy level.
Formation of ions
u
Mg
2+
+ 2e
-
Mg
O + 2e
-
O
2-
2
2
6
2
4
2
2
6
2
6
1s
2s
2p
3s
3p
1s
2s
2p
3s
3p
2
2
6
2
1s
2s
2p
3s
1s
2
2s
2
2p
6
O
2-
Mg
O
2+
-
Mg
+ 2e
Transition metals may carry different charges, become stable by
u
achieving pseudo noble-gas configuration
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12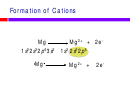 13
13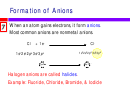 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22