7
Form 8947 (Rev. 10-2013)
Page
Section references are to the Internal Revenue Code unless otherwise
If you check the “Yes” box, you are authorizing the IRS to call the
noted.
designee to answer any questions that may arise and the designee to
call the IRS with any questions related to the administration of the 2014
Future Developments
branded prescription drug fee year. You are also authorizing the
designee to:
For the latest information about developments related to Form 8947 and
its instructions, such as legislation enacted after they were published,
• Receive copies of IRS letters upon request,
go to
• Respond to IRS letters, and
What's New
• Receive and provide information regarding the status of a payment
or an amount due back to you.
For the 2012 sales year, you can file Form 8947 as a fillable PDF
For more information, see Definitions and Item B. Covered Entity
document.
Information below. Also, see Temporary Regulations sections 51.1T
General Instructions
through 51.12T, and section 51.6302-1T.
Who Files
Purpose of Form
Generally, each manufacturer or importer of branded prescription drugs
Use Form 8947 to report the following information for branded
with sales to specified government programs (or sales due to coverage
prescription drugs sold by covered entities to specified government
under the programs) may submit Form 8947. Each entity that is treated
programs (or sales due to coverage under the programs) during sales
as a single covered entity is requested to file one Form 8947, providing
year 2012.
all requested information for each such manufacturing and reporting
• National Drug Codes (NDCs).
entity, as described in these instructions.
• Medicaid state supplemental rebate information.
Schedules A, B, C, D, and E. All filers must complete page 1 and page
6, which includes Schedule E, Summary of Form 8947, and Part II,
• Section 45C orphan drug information.
Signature of Official Signing On Behalf of the Covered Entity (Single-
• Designated entity and controlled group members information, if
Member, Common Parent of an Affiliated Group, or Other Designated
applicable.
Entity) and Consent by the Common Parent or Designated Entity (if
The IRS will use the information you submit on Form 8947 to calculate
applicable).
the annual fee for branded prescription drug sales ("the fee"). The fee is
First time filers must also attach Schedule A, Branded Prescription
imposed by section 9008 of Public Law 111-148 (Patient Protection and
Drug Information—First Time Filers Only.
Affordable Care Act), as amended by Public Law 111-152 (Health Care
Subsequent year filers with changes to report must attach Schedule
and Education Reconciliation Act of 2010) (the “Act”).
B, Branded Prescription Drug Information NDC Additions and Deletions,
If you want to designate your employee to discuss your report with
or Schedule C, Branded Prescription Drug Information Orphan Drug
the IRS, check the "Yes" box on page 6, under Alternate Contact Person
Changes—Previously Reported NDCs, or both.
Designee. Also, enter the designee's name, title, and phone number.
Completing Pages 1 and 6, and the Correct Schedule(s)
First time filer
Subsequent year filer with
Subsequent year filer with no
Subsequent year filer with no
(check Item A, box 1)
changes
changes, reporting rebates
changes, not reporting rebates
(check Item A, box 2)
(check Item A, box 3)
(check Item A, box 4)
Page 1
Yes
Yes
Yes
Yes
Schedule A
Yes
No
No
No
Yes, if NDC additions or
Schedule B
No
deletions (1), (2), (3)
No
No
Schedule C
No
Yes, if orphan drug changes (1), (3)
No
No
Schedule D
No
Yes, if reporting rebates (1), (3)
Yes
No
Schedule E
Yes, if Item B, box 2a or 2b,
Yes, if Item B, box 2a or 2b,
Yes, if Item B, box 2a or 2b,
Yes, if Item B, box 2a or 2b,
Schedule E, Line 1
checked
checked
checked
checked
Schedule E, Line 2
Yes
No
No
No
Schedule E, Line 3
Yes
No
No
No
Schedule E, Line 4
No
Yes, if Schedule B attached
No
No
Schedule E, Line 5
No
Yes, if Schedule B attached
No
No
Schedule E, Line 6
No
Yes, if Schedule B attached
No
No
Schedule E, Line 7
No
Yes, if Schedule C attached
No
No
Schedule E, Line 8
No
Yes, if Schedule D attached
Yes
No
Schedule E, Line 9
No
Yes, if Schedule D attached
Yes
No
Part II
Yes
Yes
Yes
Yes
(1) NDCs reported on Schedule B cannot be shown on Schedules C or D.
(2) On Schedule B, Section I, report as additions only NDCs that were not associated with the covered entity for the previous sales year.
(3) On Schedule B, Section II: Schedule C; or Schedule D, report only NDCs that were associated with the covered entity for the previous sales year.
 1
1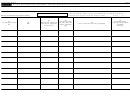 2
2 3
3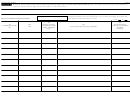 4
4 5
5 6
6 7
7 8
8 9
9








