Chem101 Molecular Models Worksheet By Dr. Cheng-Yu Lai With Answer Key Page 8
ADVERTISEMENT
2014Spring CHEM101 Ch9-10 Review Worksheet
Modified by Dr. Cheng-Yu Lai,
C)
D)
E)
Answer: C
29) Draw the Lewis structure for CO 3 2- including any valid resonance structures. Which of the following
statements is TRUE?
A) The CO 3 2- ion contains one C—O single bond and two C=O double bonds.
B) The CO 3 2- ion contains two C—O single bonds and one C=O double bond.
C) The CO 3 2- ion contains three C—O double bonds.
D) The CO 3 2- ion contains two C—O single bonds and one C≡O triple bond.
E) None of the above are true.
Answer: B
30) Using Lewis structures and formal charge, which of the following ions is most stable?
OCN⁻
ONC⁻
NOC⁻
A) OCN⁻
B) ONC⁻
C) NOC⁻
D) None of these ions are stable according to Lewis theory.
E) All of these compounds are equally stable according to Lewis theory.
Answer: A
31) Rank the following molecules in decreasing bond energy.
Cl 2
Br 2
F 2
I 2
A) I 2 > Br 2 > Cl 2 > F 2
B) Cl 2 > Br 2 > F 2 > I 2
C) I 2 > Cl 2 > Br 2 > F 2
D) Cl 2 > I 2 > F 2 > Br 2
Answer: B
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5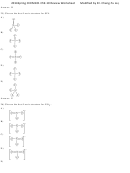 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15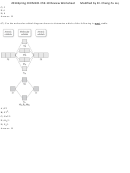 16
16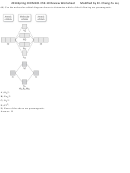 17
17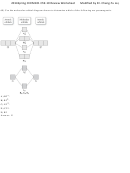 18
18








