Chem101 Molecular Models Worksheet By Dr. Cheng-Yu Lai With Answer Key Page 9
ADVERTISEMENT
2014Spring CHEM101 Ch9-10 Review Worksheet
Modified by Dr. Cheng-Yu Lai,
32) Use the bond energies provided to estimate ΔH° rxn for the reaction below.
PCl 3 (g) + Cl 2 (g) → PCl 5 (l) ΔH° rxn = ?
Bond
Bond Energy (kJ/mol)
Cl-Cl
243
P-Cl
331
A) -243 kJ
B) -419 kJ
C) -662 kJ
D) -67 kJ
E) -905 kJ
Answer: B
33) Use the bond energies provided to estimate ΔH° rxn for the reaction below.
CH 3 OH(l) + 2 O 2 (g) → CO 2 (g) + 2 H 2 O(g)
ΔH° rxn = ?
Bond
Bond Energy (kJ/mol)
C-H
414
C-O
360
C=O
799
O=O
498
O-H
464
A) +473 kJ
B) -91 kJ
C) -486 kJ
D) -392 kJ
E) +206 kJ
Answer: D
34) Use the bond energies provided to estimate ΔH° rxn for the reaction below.
XeF 2 + 2 F 2 → XeF 6
ΔH° rxn = ?
Bond
Bond Energy (kJ/mol)
Xe-F
147
F-F
159
A) -429 kJ
B) +159 kJ
C) -660 kJ
D) +176 kJ
E) -270 kJ
Answer: E
Ch10
35) Give the approximate bond angle for a molecule with an octahedral shape.
A) 109.5°
B) 180°
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5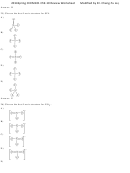 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15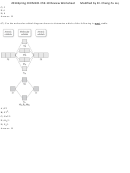 16
16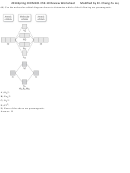 17
17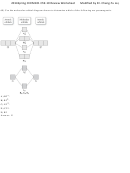 18
18








