Covalent Molecules And Lewis Structures Worksheet With Answers Page 7
ADVERTISEMENT
Part III: Analyze and Conclude
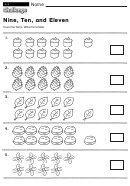
1. How many covalent bonds form around each of the following atoms:
a. hydrogen ___________! !
!
d. nitrogen
___________
b. carbon
___________! !
!
e. chlorine
___________
c. oxygen
___________! !
!
f. bromine
___________
2. In a molecular model, a single gray link (single bond) between two elements
represents ______ electrons.
3. Identify the type of bond described for each of the following as ionic, polar covalent, or
nonpolar covalent.
!
_____ a. The C–O bonds in CO
!!
!
_____ b. The bonds in F
2
2
!
!
_____ c. The bonds in K
O! !
!
!
_____ d. The C–C bonds in C
H
2
3
8
4. How many unshared pairs of electrons are found on the nitrogen atom in NH
? ______
3
5. How many electrons are shared in the triple bond in N
?
2
_____________________________________________________________________
6. Water and carbon dioxide molecules are both made up of 3 atoms. Referring to Data
Table 4, state several differences between water molecules and carbon dioxide
molecules.
_____________________________________________________________________
_____________________________________________________________________
7. How many double bonds does carbon dioxide contain? ________________________
8. Does the carbon atom in carbon dioxide have an octet of electrons? ______________
9. Which of the following elements (C, H, O, or N) will NOT be surrounded by an octet of
electrons in a correctly drawn Lewis structure? _______________________________
10. Which of the diatomic elements (N
, O
, F
, or Br
) has a double bond between its
2
2
2
2
atoms? _____________________________________________________________
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7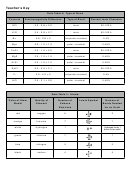 8
8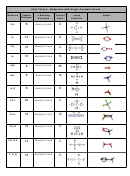 9
9 10
10