Chemistry Chem 125, 126, 130 Homework 4 With Answers - University Of Michigan, Fall 2006 Page 3
ADVERTISEMENT
Chem 125, 126, 130
Fall 2006
HW 4
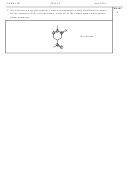
2) Find the error in each of the following Lewis structures. Circle the error and
redraw the structure so that it is correct.
H
H
H
C
C
H
H
C
C
H
H
O
C
O
H
-1
-1
O
H
H
H
C
C
H
H
O
C
O
H
C
C
H
H
O
3) Identify two elements that can represent “X” if X has one lone pair in the
molecule XBr
.
4
Group 6: S, Se
Moore, Stanitski, and Jurs: Chapter 8: 35 (explain why), 39, 64, 79, 85, 91, 95
Chapter 9: 22, 26
35 a) B-Cl; Boron has a smaller atomic radius than gallium
b) C-O; Carbon has a smaller atomic radius than tin
c) P-O; Oxygen has a smaller atomic radius than sulfur
d) C=O Oxygen has a smaller atomic radius than carbon
39)
O
C≡O of carbon monoxide takes more energy to break
C
O
than the C=O of formaldehyde
C
H
H
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3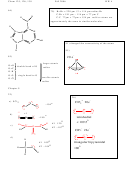 4
4