Molecular Geometry, Polarity, Hybridization Worksheet
ADVERTISEMENT
GAGE
CHM 1010
Worksheet for Molecular Geometry, Polarity, Hybridization
1.
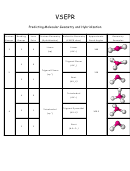
Complete the following table. Use N/A where something does not apply.
Please use dots for all pairs; because of the drawing
application used the bonds cannot be displayed as electron pairs on this answer sheet
Compound/Ion
Dot Structure
Molecular Geometry
Polar or Non-Polar
Type of Hybridization
(name only, no drawings)
Molecule?
3
NHCl
trigonal pyramidal
polar, electron pair
sp
2
Cl
and chlorines are
N
Cl
negative; H is positive
H
MgS
N/A, ionic so ions are
N/A, ionic, not
N/A
separate
covalent molecule so
no electron pair
sharing
3
CH
F
tetrahedral
polar with F as
sp
H
3
negative end of dipole
H
C
F
H
3
3
ICl
T-shaped
polar with electron
dsp
or sp
d
3
Cl
pairs as negative end
of dipole
I
Cl
Cl
2-
2
SiO
trigonal planar
N/A, ion with charge
sp
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2