Solving Stoichiometry Problem Worksheet With Answers Page 8
ADVERTISEMENT
Step 4: Lets Set up the Equation
The problem states If you start with 10.0gm of Lithium hydroxide,
how many grams of lithium bromide will be produced?
We know that 1mole of any
Set up the T-Chart
molecule is equal to the molar
1 mol LiOH
mass of that molecule:
10 gm LiOH
So on top of the 24gm of LiOH, I
24gm LiOH
will have 1mole of LiOH
But I need to solve for LiBr, so I
need to cancel my mole of LiOH
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11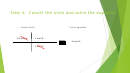 12
12








