Solving Stoichiometry Problem Worksheet With Answers Page 9
ADVERTISEMENT
Step 5: Look at my Mole Ratio
The problem states If you start with 10.0gm of Lithium hydroxide,
how many grams of lithium bromide will be produced?
As stated I need to solve for LiBr, so I need
Set up the T-Chart
to cancel my mole of LiOH
So now I will look at my mole ratio
1 mol of LiBr
10 gm LiOH
1 mol LiOH
1LiOH + 1HBr
1 LiBr + 1H
O
2
24gm LiOH
1mol LiOH
1: 1: 1: 1
I have 1 mole of LiOH based off my ratio
I place that on the bottom so that I can
cancel LiOH
Then I look at what I need, I need grams
of LiBr
But I have 1 mole of LiBr so I can place
that on top
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11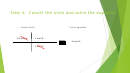 12
12








