Recrystallization Page 2
ADVERTISEMENT
2
Part 2. Solubility Tests
In the second part of today's experiment, you will test the solubility properties of
resorcinol and benzoic acid (see structures below) in seven different solvents: methanol,
ethanol, acetone, acetic acid, water, toluene and ligroin (also known as petroleum ether).
See LTOC, Section 15.4, and the table on page 222. Knowing the solubility properties,
you will be able to draw some conclusions regarding an appropriate recrystallization
solvent or solvents for each compound.
OH
O
C
OH
OH
Resorcinol
Benzoic acid
Use a flow chart format to outline the steps of the following procedure (see below
for an example). You should perform these tests while your phthalic acid is crystallizing.
Mix 20-30 mg of solid
with methanol. Stir!
soluble?
no
yes
Add a few drops of water
Heat mixture in water bath
precipitate?
no
yes
etc.
etc.
etc.
Caution! Methanol, ethanol, acetone, toluene and ligroin are flammable!
To test the solubility of a solid, transfer a “spatula-tip” full of the compound (you
may weigh out 20-30 mg of one of the solids to get an idea of how much should be used)
into a 10x75 mm test tube and add 10 drops of solvent. Stir with a thin stirring rod, break
up any lumps, observe and record whether or not the solid is readily soluble at room
temperature.
If the solid does not dissolve in a given solvent at room temperature, heat the test
tube containing the mixture in a beaker of water heated on a hot plate. If the solid
ADVERTISEMENT
0 votes
Related Articles
Related Categories
Parent category: Education
 1
1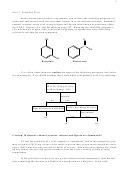 2
2 3
3 4
4