Periodic Trends Worksheet
ADVERTISEMENT
Date:
Periodic Trends
1.
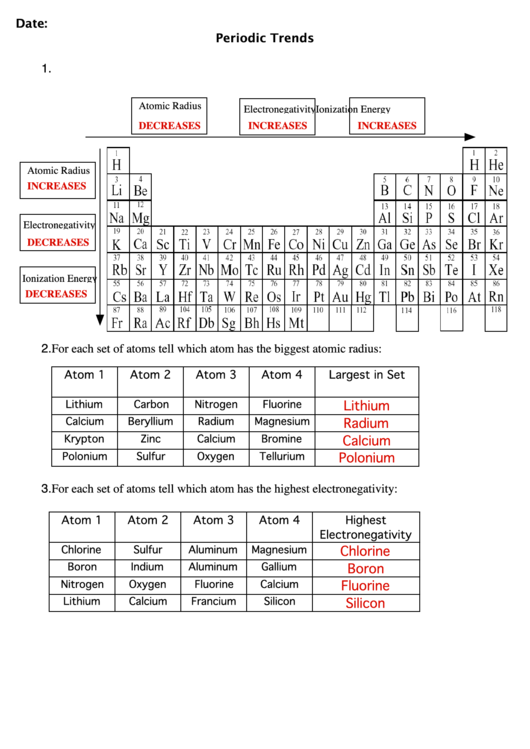
Atomic Radius
Electronegativity
Ionization Energy
DECREASES
INCREASES
INCREASES
Atomic Radius
INCREASES
Electronegativity
DECREASES
Ionization Energy
DECREASES
2.For each set of atoms tell which atom has the biggest atomic radius:
Atom 1
Atom 2
Atom 3
Atom 4
Largest in Set
Lithium
Carbon
Nitrogen
Fluorine
Lithium
Calcium
Beryllium
Radium
Magnesium
Radium
Krypton
Zinc
Calcium
Bromine
Calcium
Polonium
Sulfur
Oxygen
Tellurium
Polonium
3.For each set of atoms tell which atom has the highest electronegativity:
Atom 1
Atom 2
Atom 3
Atom 4
Highest
Electronegativity
Chlorine
Sulfur
Aluminum
Magnesium
Chlorine
Boron
Indium
Aluminum
Gallium
Boron
Nitrogen
Oxygen
Fluorine
Calcium
Fluorine
Lithium
Calcium
Francium
Silicon
Silicon
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








