Better Understanding Of Element Property Trends
ADVERTISEMENT
Better Understanding of Element Property Trends
Description of the TrendsTube
Teaching Aid Rationale & Product
®
Property trend patterns - physical, electrical, or chemical aspects of adjacent
elements of the periodic table - increase or
decrease in different directions, but often not
strictly continuous. The main trends are:
ionization energy, electron affinity, metallic
character, atomic radius, electronegativity,
and melting point. Chemists may use them to
predict an element's properties by its position
relative to others, a common application of the
periodic table in general.
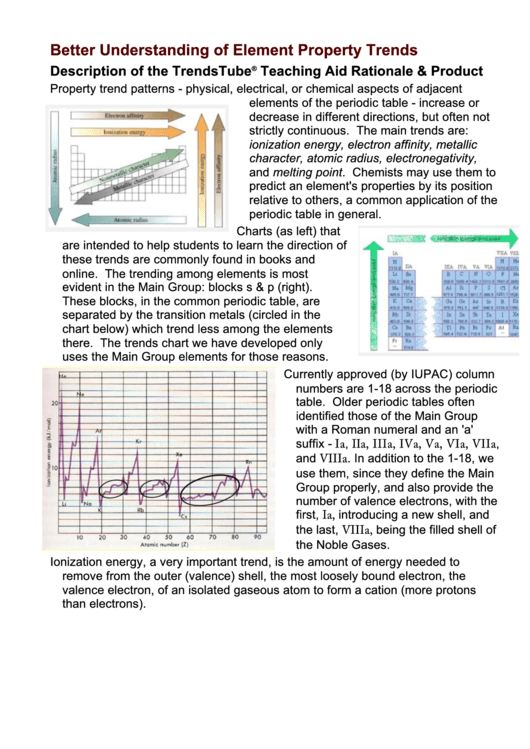
Charts (as left) that
are intended to help students to learn the direction of
these trends are commonly found in books and
online. The trending among elements is most
evident in the Main Group: blocks s & p (right).
These blocks, in the common periodic table, are
separated by the transition metals (circled in the
chart below) which trend less among the elements
there. The trends chart we have developed only
uses the Main Group elements for those reasons.
Currently approved (by IUPAC) column
numbers are 1-18 across the periodic
table. Older periodic tables often
identified those of the Main Group
with a Roman numeral and an 'a'
suffix - Ia, IIa, IIIa, IVa, Va, VIa, VIIa,
and VIIIa. In addition to the 1-18, we
use them, since they define the Main
Group properly, and also provide the
number of valence electrons, with the
first, Ia, introducing a new shell, and
the last, VIIIa, being the filled shell of
the Noble Gases.
Ionization energy, a very important trend, is the amount of energy needed to
remove from the outer (valence) shell, the most loosely bound electron, the
valence electron, of an isolated gaseous atom to form a cation (more protons
than electrons).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








