Solubility Chart Page 2
ADVERTISEMENT
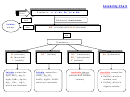
Electrolyte Strength
Acids
Bases
Ionic Salts
Strong
Weak
Non-
Strong
Weak
Non-
Strong
Weak
Non-
Electrolyte
Electrolyte
electrolyte
Electrolyte
Electrolyte
electrolyte
Electrolyte
Electrolyte
electrolyte
HCl
All other
Water
LiOH
NH
or
Insoluble
Soluble
HgCl
Insoluble
3
2
HBr
water
insoluble
NaOH
NH
OH
bases, most
salts, see
CdCl
salts
4
2
HI
soluble acids
acids
KOH
other metal
rules or
Pb(CH
CO
)
3
2
2
HNO
RbOH
Amines
hydroxides
chart
3
H
SO
CsOH
2
4
HClO
Sr(OH)
4
2
HBrO
Ba(OH)
4
2
HIO
4
HClO
3
Ions
Molecules
Solids
Ions
Molecules
Solids
Ions
Few Ions
Solids
Solubility Rules
Soluble Compounds
Exceptions
Insoluble Compounds
Exceptions
Nitrates
None
Carbonates
Alkali metals, ammonium
Chlorates
None
Phosphates
Alkali metals, ammonium
Perchlorates
K is slightly soluble
Chromates
Alkali metals, ammonium
Acetates
Ag and Hg(I) are slightly soluble
Oxalates
Alkali metals, ammonium
Chlorides, Bromides,
Ag , Pb(II), and Hg(I) are insoluble
Sulfites
Alkali metals, ammonium
Iodides
Fluorides
Ca, Mg, Sr, Ba, and Pb(II)
Sulfides
Alkali metals, ammonium, Ca, Sr,
Ba
Sulfates
Sr, Ba , Pb(II), and Hg(I) are insoluble;
Oxides
Li, Na, Ca, Sr, Ba
Ag and Ca are slightly soluble
Alkali Metals
KClO
(see above)
Hydroxides
Alkali metals, ammonium, Sr, Ba;
4
Ca is slightly soluble
Ammonium
None
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2