Solubility Chart Template
ADVERTISEMENT
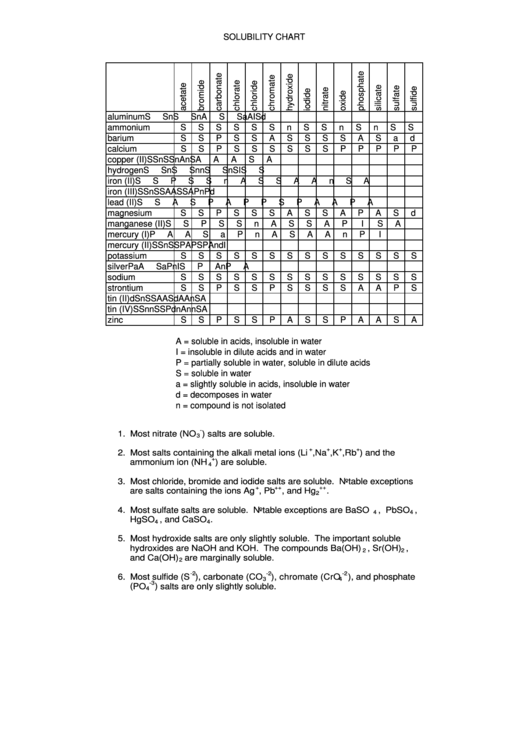
SOLUBILITY CHART
aluminum
S
S
n
S
S
n
A
S
S
a
A
I
S
d
ammonium
S
S
S
S
S
S
n
S
S
n
S
n
S
S
barium
S
S
P
S
S
A
S
S
S
S
A
S
a
d
calcium
S
S
P
S
S
S
S
S
S
P
P
P
P
P
copper (II)
S
S
n
S
S
n
A
n
S
A
A
A
S
A
hydrogen
S
S
n
S
S
n
n
S
S
n
S
I
S
S
iron (II)
S
S
P
S
S
n
A
S
S
A
A
n
S
A
iron (III)
S
S
n
S
S
A
A
S
S
A
P
n
P
d
lead (II)
S
S
A
S
P
A
P
P
S
P
A
A
P
A
magnesium
S
S
P
S
S
S
A
S
S
A
P
A
S
d
manganese (II)
S
S
P
S
S
n
A
S
S
A
P
I
S
A
mercury (I)
P
A
A
S
a
P
n
A
S
A
A
n
P
I
mercury (II)
S
S
n
S
S
P
A
P
S
P
A
n
d
I
potassium
S
S
S
S
S
S
S
S
S
S
S
S
S
S
silver
P
a
A
S
a
P
n
I
S
P
A
n
P
A
sodium
S
S
S
S
S
S
S
S
S
S
S
S
S
S
strontium
S
S
P
S
S
P
S
S
S
S
A
A
P
S
tin (II)
d
S
n
S
S
A
A
S
d
A
A
n
S
A
tin (IV)
S
S
n
n
S
S
P
d
n
A
n
n
S
A
zinc
S
S
P
S
S
P
A
S
S
P
A
A
S
A
A = soluble in acids, insoluble in water
I = insoluble in dilute acids and in water
P = partially soluble in water, soluble in dilute acids
S = soluble in water
a = slightly soluble in acids, insoluble in water
d = decomposes in water
n = compound is not isolated
-
1. Most nitrate (NO
) salts are soluble.
3
+
+
+
+
2. Most salts containing the alkali metal ions (Li
,Na
,K
,Rb
) and the
+
ammonium ion (NH
) are soluble.
4
3. Most chloride, bromide and iodide salts are soluble. Notable exceptions
+
++
++
are salts containing the ions Ag
, Pb
, and Hg
.
2
4. Most sulfate salts are soluble. Notable exceptions are BaSO
, PbSO
,
4
4
HgSO
, and CaSO
.
4
4
5. Most hydroxide salts are only slightly soluble. The important soluble
hydroxides are NaOH and KOH. The compounds Ba(OH)
, Sr(OH)
,
2
2
and Ca(OH)
are marginally soluble.
2
-2
-2
-2
6. Most sulfide (S
), carbonate (CO
), chromate (CrO
), and phosphate
3
4
-3
(PO
) salts are only slightly soluble.
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1