Solubility Table
ADVERTISEMENT
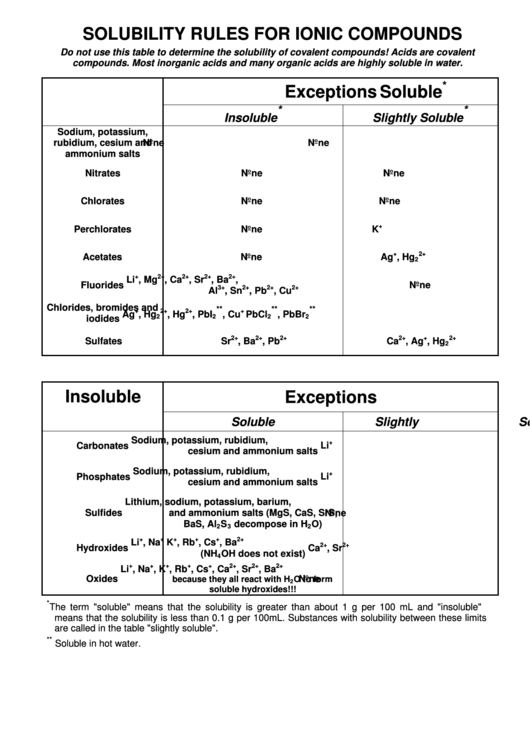
SOLUBILITY RULES FOR IONIC COMPOUNDS
Do not use this table to determine the solubility of covalent compounds! Acids are covalent
compounds. Most inorganic acids and many organic acids are highly soluble in water.
*
Soluble
Exceptions
*
*
Insoluble
Slightly Soluble
Sodium, potassium,
rubidium, cesium and
None
None
ammonium salts
Nitrates
None
None
Chlorates
None
None
+
Perchlorates
None
K
+
2+
Acetates
None
Ag
, Hg
2
+
2+
2+
2+
2+
Li
, Mg
, Ca
, Sr
, Ba
,
Fluorides
None
3+
2+
2+
2+
Al
, Sn
, Pb
, Cu
Chlorides, bromides and
**
**
**
+
2+
2+
+
Ag
, Hg
, Hg
, PbI
, Cu
PbCl
, PbBr
2
2
2
2
iodides
2+
2+
2+
2+
+
2+
Sulfates
Sr
, Ba
, Pb
Ca
, Ag
, Hg
2
Insoluble
Exceptions
Soluble
Slightly Soluble
Sodium, potassium, rubidium,
+
Carbonates
Li
cesium and ammonium salts
Sodium, potassium, rubidium,
+
Phosphates
Li
cesium and ammonium salts
Lithium, sodium, potassium, barium,
Sulfides
and ammonium salts (MgS, CaS, SrS,
None
BaS, Al
S
decompose in H
O)
2
3
2
+
+
+
+
+
2+
Li
, Na
K
, Rb
, Cs
, Ba
2+
2+
Hydroxides
Ca
, Sr
(NH
OH does not exist)
4
+
+
+
+
+
2+
2+
2+
Li
, Na
, K
, Rb
, Cs
, Ca
, Sr
, Ba
Oxides
None
because they all react with H
O to form
2
soluble hydroxides!!!
*
The term "soluble" means that the solubility is greater than about 1 g per 100 mL and "insoluble"
means that the solubility is less than 0.1 g per 100mL. Substances with solubility between these limits
are called in the table "slightly soluble".
**
Soluble in hot water.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








