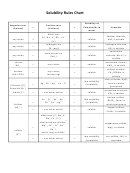
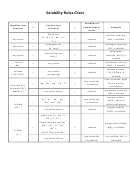
Solubility Rules Chart
ADVERTISEMENT
Chem 115
Zamis
Solubility Rules
SOLUBLE COMPOUNDS
INSOLUBLE COMPOUNDS
2-
compounds of Group 1 elements
carbonates(CO
) except those of Group I
3
+
elements and NH
4
+
2-
ammonium(NH
) compounds
oxalates(C
O
) except those of Group I
4
2
4
+
elements and NH
4
-
-
-
3-
chlorides(Cl
), bromides(Br
), iodides(I
) except
phosphates(PO
) except those of Group I
4
+
2+
2+
+
those of Ag
, Hg
, and Pb
elements and NH
2
4
-
-
2-
nitrates(NO
), acetates(C
H
O
),
sulfides(S
) except those of Group 1 and 2
3
2
3
2
-
-
+
chlorates(ClO
) and perchlorates(ClO
)
elements and NH
3
4
4
2-
2+
2+
2+
-
sulfates(SO
) except those of Ca
, Sr
, Ba
,
hydroxides(OH
) except those of Group 1 and
4
2+
2+
+
+
Pb
, Hg
and Ag
2 elements and NH
2
4
Soluble ionic compounds in water will dissociate to give the individual cations and anions.
(Strong electrolytes)
Strong
Acids
&
Bases
in Water
STRONG ACIDS
STRONG BASES
hydrochloric acid, HCl(aq)
lithium hydroxide, LiOH (aq)
hydrobromic acid, HBr(aq)
sodium hydroxide, NaOH (aq)
hydroiodic acid, HI(aq)
potassium hydroxide, KOH (aq)
nitric acid, HNO
(aq)
Ca hydroxide, Ca(OH)
(aq)
3
2
-
sulfuric acid, H
SO
(aq) (to HSO
(aq))
Sr hydroxide, Sr(OH)
(aq)
2
4
4
2
perchloric acid, HClO
(aq)
Ba hydroxide, Ba(OH)
(aq)
4
2
Strong acids and bases in water will dissociate to give the individual cations and anions.
(Strong electrolytes)
Weak
Acids
&
Bases
in Water
WEAK ACIDS
WEAK BASES
phosphoric acid, H
PO
(aq)
ammonia, NH
(aq)
3
4
3
organic acids, R-CO
H
organic amines, R-NH
2
2
Weak acids and bases in water will partially react with water to give a small percentage of
cations and anions (weak electrolyte), but most of the compound will remain in its original form.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1