Hs Chemistry Teacher Goal Setting Example Template
ADVERTISEMENT
TEACHER SLG GOAL SETTING EXAMPLE – HIGH SCHOOL CHEMISTRY
Grade Level:
Elementary
Middle School
High School
Goal Type:
Individual Goal
Team Goal
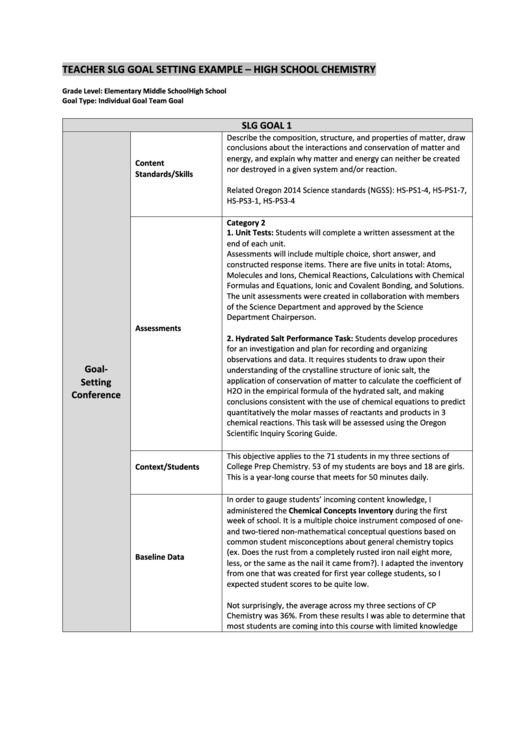
SLG GOAL 1
Describe the composition, structure, and properties of matter, draw
conclusions about the interactions and conservation of matter and
energy, and explain why matter and energy can neither be created
Content
nor destroyed in a given system and/or reaction.
Standards/Skills
Related Oregon 2014 Science standards (NGSS): HS-PS1-4, HS-PS1-7,
HS-PS3-1, HS-PS3-4
Category 2
1. Unit Tests: Students will complete a written assessment at the
end of each unit.
Assessments will include multiple choice, short answer, and
constructed response items. There are five units in total: Atoms,
Molecules and Ions, Chemical Reactions, Calculations with Chemical
Formulas and Equations, Ionic and Covalent Bonding, and Solutions.
The unit assessments were created in collaboration with members
of the Science Department and approved by the Science
Department Chairperson.
Assessments
2. Hydrated Salt Performance Task: Students develop procedures
for an investigation and plan for recording and organizing
observations and data. It requires students to draw upon their
Goal-
understanding of the crystalline structure of ionic salt, the
application of conservation of matter to calculate the coefficient of
Setting
H2O in the empirical formula of the hydrated salt, and making
Conference
conclusions consistent with the use of chemical equations to predict
quantitatively the molar masses of reactants and products in 3
chemical reactions. This task will be assessed using the Oregon
Scientific Inquiry Scoring Guide.
This objective applies to the 71 students in my three sections of
Context/Students
College Prep Chemistry. 53 of my students are boys and 18 are girls.
This is a year-long course that meets for 50 minutes daily.
In order to gauge students’ incoming content knowledge, I
administered the Chemical Concepts Inventory during the first
week of school. It is a multiple choice instrument composed of one-
and two-tiered non-mathematical conceptual questions based on
common student misconceptions about general chemistry topics
(ex. Does the rust from a completely rusted iron nail eight more,
Baseline Data
less, or the same as the nail it came from?). I adapted the inventory
from one that was created for first year college students, so I
expected student scores to be quite low.
Not surprisingly, the average across my three sections of CP
Chemistry was 36%. From these results I was able to determine that
most students are coming into this course with limited knowledge
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Life
 1
1 2
2








