Solubility Curves Ws
ADVERTISEMENT
Solubility Curves WS
Name: _____________________
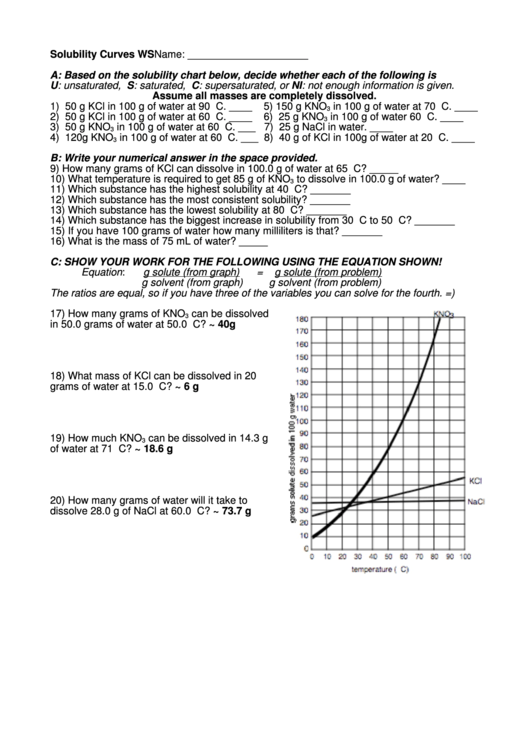
A: Based on the solubility chart below, decide whether each of the following is
U: unsaturated, S: saturated, C: supersaturated, or NI: not enough information is given.
Assume all masses are completely dissolved.
1) 50 g KCl in 100 g of water at 90 C. ____
5) 150 g KNO
in 100 g of water at 70 C. ____
3
2) 50 g KCl in 100 g of water at 60 C. ____
6) 25 g KNO
in 100 g of water 60 C. ____
3
3) 50 g KNO
in 100 g of water at 60 C. ___ 7) 25 g NaCl in water. ____
3
4) 120g KNO
in 100 g of water at 60 C. ___ 8) 40 g of KCl in 100g of water at 20 C. ____
3
B: Write your numerical answer in the space provided.
9) How many grams of KCl can dissolve in 100.0 g of water at 65 C? _____
10) What temperature is required to get 85 g of KNO
to dissolve in 100.0 g of water? ____
3
11) Which substance has the highest solubility at 40 C? _______
12) Which substance has the most consistent solubility? _______
13) Which substance has the lowest solubility at 80 C? _______
14) Which substance has the biggest increase in solubility from 30 C to 50 C? _______
15) If you have 100 grams of water how many milliliters is that? _______
16) What is the mass of 75 mL of water? _____
C: SHOW YOUR WORK FOR THE FOLLOWING USING THE EQUATION SHOWN!
Equation:
g solute (from graph)
=
g solute (from problem)
g solvent (from graph)
g solvent (from problem)
The ratios are equal, so if you have three of the variables you can solve for the fourth. =)
17) How many grams of KNO
can be dissolved
3
in 50.0 grams of water at 50.0 C? ~ 40g
18) What mass of KCl can be dissolved in 20
grams of water at 15.0 C? ~ 6 g
19) How much KNO
can be dissolved in 14.3 g
3
of water at 71 C? ~ 18.6 g
20) How many grams of water will it take to
dissolve 28.0 g of NaCl at 60.0 C? ~ 73.7 g
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








