Hardness Of Minerals Va: Variation Among Oxides And Oxysalts
ADVERTISEMENT
Railsback's Some Fundamentals of Mineralogy and Geochemistry
Hardness of minerals Va: variation among oxides and oxysalts
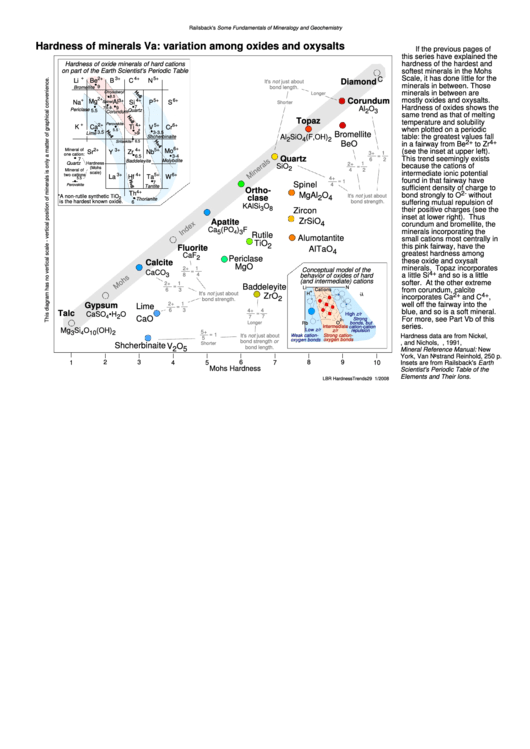
If the previous pages of
this series have explained the
hardness of the hardest and
Hardness of oxide minerals of hard cations
on part of the Earth Scientist's Periodic Table
softest minerals in the Mohs
Scale, it has done little for the
+
2+
3+
4+
5+
C
Li
Be
B
C
N
Diamond
It's not just about
minerals in between. Those
9
bond length.
Bromellite
minerals in between are
Chrysoberyl
Longer
8.5
2+
mostly oxides and oxysalts.
+
Corundum
3+
4+
5+
6+
Mg
Al
Na
Spinel
Si
P
S
Shorter
Hardness of oxides shows the
7
9
Al
O
7.5-8
Periclase
5.5
Quartz
2
3
Corundum
same trend as that of melting
Topaz
temperature and solubility
Perovskite
+
2+
4+
5+
6+
K
Ca
Ti
V
Cr
when plotted on a periodic
5.5
*
3.5
>9
3-3.5
Lime
Bromellite
Al
SiO
(F,OH)
table: the greatest values fall
Shcherbinaite
2
4
2
2+
4+
Srilankite
6.5
BeO
in a fairway from Be
to Zr
6+
Mineral of
2+
3+
4+
5+
Mo
(see the inset at upper left).
Sr
Y
Zr
Nb
1
3+
one cation:
=
6.5
3-4
Quartz
This trend seemingly exists
7
6
2
Baddeleyite
Molybdite
Quartz
Hardness
2+
1
SiO
because the cations of
=
(Mohs
2
2
Mineral of
4
intermediate ionic potential
scale)
two cations
3+
4+
5+
6+
La
Hf
Ta
W
5.5
4+
found in that fairway have
= 1
7
Spinel
4
Perovskite
Tantite
sufficient density of charge to
Ortho-
4+
Th
2-
MgAl
O
bond strongly to O
without
It's not just about
*A non-rutile synthetic TiO
clase
2
4
2
Thorianite
is the hardest known oxide.
bond strength.
suffering mutual repulsion of
6
KAlSi
O
3
8
their positive charges (see the
Zircon
inset at lower right). Thus
ZrSiO
Apatite
4
corundum and bromellite, the
Ca
(PO
)
F
minerals incorporating the
5
3
4
Rutile
Alumotantite
small cations most centrally in
TiO
2
this pink fairway, have the
Fluorite
AlTaO
4
greatest hardness among
CaF
2
Periclase
these oxide and oxysalt
Calcite
MgO
minerals. Topaz incorporates
1
2+
Conceptual model of the
=
CaCO
4+
3
4
a little Si
and so is a little
8
behavior of oxides of hard
(and intermediate) cations
softer. At the other extreme
2+
1
Baddeleyite
=
N
Li
from corundum, calcite
3
Cations
6
+
It's not just about
H
ZrO
2+
4+
1Å
incorporates Ca
and C
,
2
bond strength.
2+
1
well off the fairway into the
Gypsum
Lime
=
6
3
4+
4
blue, and so is a soft mineral.
Talc
CaSO
•H
O
=
High z/r
2
4
7
7
CaO
For more, see Part Vb of this
Strong
2–
Longer
O
bonds, but
Rb
series.
Intermediate
cation-cation
Mg
Si
O
(OH)
Low z/r
repulsion
z/r
5+
3
10
2
4
= 1
Hardness data are from Nickel,
It's not just about
Weak cation-
Strong cation-
5
oxygen bonds
oxygen bonds
bond strength or
E.H., and Nichols, M.C., 1991,
Shorter
Shcherbinaite
V
O
bond length.
2
5
Mineral Reference Manual: New
York, Van Nostrand Reinhold, 250 p.
2
3
6
8
9
1
4
5
7
10
Insets are from Railsback's Earth
Mohs Hardness
Scientist's Periodic Table of the
Elements and Their Ions.
LBR HardnessTrends29 1/2008
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Miscellaneous
 1
1








