Benzene: Aromatic Hydrocarbons Sheet Template Page 3
ADVERTISEMENT
Friedel Crafts Acylation
Change in functional group: benzene
phenyl ketone
Any acyl chloride can be used RCOCl where
Reagents: acyl chloride in the presence of anhydrous
R is any alkyl group e.g. –CH
, -C
H
. The
aluminium chloride catalyst
3
2
5
+
electrophile is the RCO
.
O
Conditions: heat under reflux (50
C)
Mechanism: Electrophilic Substitution
Equation for Formation of the electrophile.
+
-
AlCl
+ CH
COCl
CH
CO
AlCl
3
3
3
4
Overall Equation for reaction
These are important
O
reactions in organic
C
synthesis because they
CH
3
introduce a reactive
+
-
CH
CO
AlCl
+
+ AlCl
+ HCl
3
4
3
functional group on to the
benzene ring
phenylethanone
O
Mechanism
O
+
O
C
CH
C
CH
3
3
C
CH
H
3
+
+
-
The H
ion reacts with the AlCl
to
+
-
4
H
+ AlCl
AlCl
+ HCl
4
3
reform AlCl
catalyst and HCl.
3
Reducing a nitroarene to aromatic amines
The nitro group on an arene can be reduced an amine group as follows
NO
NH
2
Reagent: Sn and HCl or Fe and HCl
2
Conditions: Heating
+ 6[H]
+ 2H
O
2
Mechanism:reduction
phenylamine
nitrobenzene
This reduction reaction can
+
-
As the reaction is carried out in HCl the salt C
H
NH
Cl
will be formed.
also be done with catalytic
6
5
3
Reacting this salt with NaOH will give phenylamine.
hydrogenation (H
using a
2
Ni catalyst)
Effect of delocalisation on side groups with lone pairs
If a –OH group, a Cl atom or an NH
group is directly attached to a benzene ring the
2
delocalisation in the benzene ring will extend to include the lone pairs on the N,O and Cl. This
changes the properties and reactions of the side group.
Cl
NH
OH
2
phenylamine
chlorobenzene
phenol
The C-Cl bond is made
stronger. Typical haloalkane
Delocalisation makes the C-O
Less basic than aliphatic
substitution and elimination
bond stronger and the O-H bond
amines as lone pair is
reactions do not occur. Also
weaker. Phenol does not act like
delocalised and less
the electron rich benzene
an alcohol- it is more acidic and
available for accepting a
ring will repel nucleophiles.
does not oxidise.
proton.
N Goalby
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3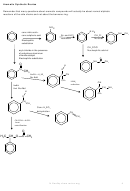 4
4








