Chemistry Organic Functional Groups
ADVERTISEMENT
24.1 (and heating food substances lab)
What evidence do we have that
When most foods are heated they turn black. This indicates the
carbon is present in most foods?
presence of carbon.
Why is the term “organic” chemistry
It suggests that all organic compounds come from living things.
misleading?
Organic compounds may be synthetic or may arise without life.
Which compounds are inorganic and
Inorganic: the oxides of carbon (CO, CO
), the bicarbonates
2
-
which are organic?
(containing the HCO
ion, such as NaHCO
), and carbonates of
3
3
2-
metal ions (compounds containing the CO
ion, such as
3
Na
CO
). All other carbon-containing compounds are organic.
2
3
Give examples of organic
Plastics, synthetic and natural fibers (like cotton and wood), most
compounds.
drugs, perfumes, petroleum products, carbohydrates, fats and oils,
proteins, vitamins, hair, cell membranes and cell components, etc.
Define functional group.
The name for the part of an organic molecule where most
chemical reactions occur.
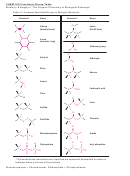
Draw the characteristic structural feature of these functional groups:
C–C
Alkanes
Alkenes
C=C
Alkynes
C≡C
H
Aromatics
H
C
H
C
C
C
C
H
C
H
or
or
(all the same)
H
R–OH
Alcohols
O
Aldehydes
(H)R
C
H
O
Ketones
R
C
R'
O
Carboxylic acids
(H)R
C
OH
Also symbolized as COOH
O
Esters
(H)R
C
O
R'
Also symbolized as COOC
R–O–R’
Ethers
R''
H
H
Amines
R
N
R'
R
N
R
'
R
N
H
or
or
(3 possibilities)
O
R''(H)
Amides
(H)R
C
N
R'(H)
Why are compounds classified
Because compounds with similar functional groups undergo
according to functional groups?
similar reactions (we only have to know a few general reactions).
The more polar the group, the higher the boiling point and the greater
How does the presence of functional
the solubility in water. For example CH
–O–CH
has a lower boiling
groups influence the boiling point
3
3
point and lower solubility in water than CH
CH
–OH (the electro-
and the solubility of molecules?
3
2
negativity difference between C and O is 1.0 versus 1.4 for H and O).
Draw a hydroxyl group
–OH
O
Draw a carbonyl group
R
C
R
O
Draw a carboxyl group
R
C
OH
You should know the rules for naming hydrocarbons (see handout)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1